Aaron M. Joffe
I. Acid–Base Interpretation and Treatment
A. Overview of Acid–Base Equilibrium
Precise regulation of blood pH is necessary for maintaining physiologic homeostasis. Outside of the normal physiologic range (7.35 to 7.45), vital functions such as oxygen transport, organ perfusion, and cellular metabolism may become impaired. At extremes of pH (<6.8 or >7.8), basic cellular processes are so impaired as to be incompatible with life.
The body is presented with significant acid and alkali loads daily, largely a consequence of nutrient intake and cellular metabolism. Nonetheless, the blood pH remains stable through buffering of hydrogen ions (H+) in the blood, their excretion by the kidneys (see Chapter 5), and elimination of carbon dioxide (CO2) by the lungs (see Chapter 2). The amount of H+ in the blood is determined by the ratio of CO2 and bicarbonate (hydrogen carbonate, HCO3−) as represented by the Henderson-Hasselbalch equation:

Accumulation of H+ or HCO3− due to exhaustion of body buffers or dysregulation by the kidneys results in metabolic disturbances, whereas high or low arterial CO2 results from respiratory disturbances. Note the semantic difference between an “–emia” and an “–osis.” Acidemia and alkalemia refer to a low or high blood pH, respectively. Acidosis or alkalosis refers to the primary processes responsible for the alterations in pH (Fig. 23-1). Only one –emia can ever be present at one time, while more than one –osis can coexist.

Acidemia and alkalemia refer to a low or high blood pH irrespective of the cause of the acid–base disturbance. Acidosis or alkalosis refers to the primary processes responsible for the alterations in pH, such as metabolic acidosis or respiratory alkalosis.
B. Metabolic Acidosis
Primary metabolic acidosis is characterized by an arterial pH <7.35 and HCO3− <22 mEq/L and occurs as a result of either accumulation of H+ or a loss of HCO3−. The nature of the acidosis can be further characterized by the presence or absence of a greater than expected concentration of unmeasured anions (high gap or normal gap, respectively). Because the plasma normally contains more unmeasured anions than cations, an anion gap (AG) in the 6 to 11 mEq/L range is normally present. Calculation of the AG is determined with the following equation:
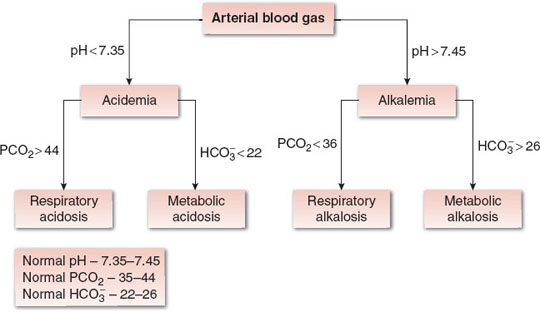
FIGURE 23-1 Derangements in acid–base status can be derived from arterial blood gas analysis by first determining acidemia or alkalemia from the pH, and then assessing the respiratory and metabolic components of the derangement from the PCO2 and HCO3− values (see text and Table 23-6 for details). PCO2, partial pressure of carbon dioxide; HCO3−, hydrogen carbonate.
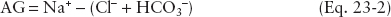
where Na+ is the sodium ion and Cl− is the chloride ion.
An elevated AG develops when an acid accumulates and then dissociates into a proton (H+) and the unmeasured anion (UA−). The proton is titrated by HCO3−, decreasing its concentration, while the UA− remains in the plasma. Because neither the Na+ nor Cl− change, the AG increases (Eqs. 23-2 and 23-3):
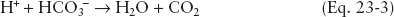
The most common causes of high AG metabolic acidosis (HAGMA) include ketoacidosis, uremia, lactic acidosis, and a variety of toxins including methanol, salicylates, paraldehydes, and ethylene glycol (Table 23-1). Nonanion gap metabolic acidosis (NAGMA) results when HCO3− is lost from the gastrointestinal tract or kidneys or due to an inability of the kidneys to excrete protons. Because electroneutrality is maintained by Cl− retention, the AG remains unchanged. The most common causes of NAGMA are excessive administration of 0.9% NaCl solution, diarrhea, and renal tubular acidosis (Table 23-1).
Table 23-1 Causes of Metabolic Acidosis
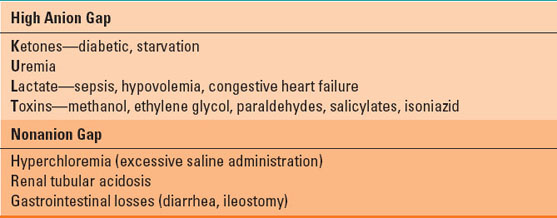
Treatment of metabolic acidosis should be directed toward correcting the underlying cause. For example, in the case of a NAGMA due to 0.9% NaCl administration, the Cl− load can be reduced with balanced lactate solutions for resuscitation or by adding 150 mL of 8.4% NaHCO3 to a 1,000-mL bag of 5% dextrose in water. Antidiarrheal medications can be given, and in cases of severe diarrhea where HCO3− losses are significant, administration of sodium bicarbonate can be considered. Treatment for HAGMA is based on the underlying cause. Ketoacidosis should be treated with insulin therapy, and lactic acidosis is treated with oxygenation, resuscitation, and cardiovascular support. Treatment of lactic acidosis with sodium bicarbonate is not recommended unless pH is <7.15 and the patient is clinically deteriorating (1,2).
C. Metabolic Alkalosis
Metabolic alkalosis is characterized by an arterial pH >7.45 (alkalemia) and HCO3− >26 mEq/L. This disorder results from a net gain of HCO3− or loss of H+ ions. The kidney has a tremendous ability to excrete HCO3−, so metabolic alkalosis must not only be generated but also maintained, usually by obligatory NaHCO3− reabsorption in the proximal tubule in the setting of hypovolemia. Severe hypokalemia from any cause may also lead to HCO3− retention. Loss of H+ ions usually results from significant vomiting or renal excretion, as suggested by a history of vomiting or diuretic use. Urine electrolytes (notably urine Cl−) are used to further characterize the metabolic alkalosis. Low urine Cl− is considered saline responsive, whereas normal or high urine Cl− is saline unresponsive. The most common causes of metabolic alkalosis are listed in Table 23-2.
Table 23-2 Causes of Metabolic Alkalosis
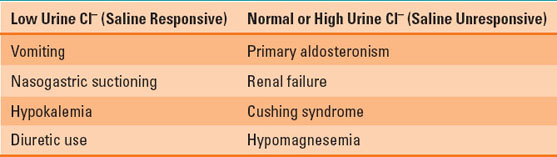
Treatment of metabolic alkalosis is based on correcting the underlying cause. Saline responsive metabolic alkalosis should receive resuscitation with sodium and potassium chloride to enable to kidneys to resume HCO3− excretion.
D. Respiratory Acidosis
Respiratory acidosis is defined as an arterial pH <7.35 and partial pressure of CO2 (PCO2) >44 mm Hg. An increase in CO2 will result in more H+ ions, decreasing the pH (Eq. 23-3). Arterial CO2 levels reflect the balance between CO2 production via cellular respiration and its excretion through alveolar ventilation. It should be noted that increased production alone would rarely be the cause of respiratory acidosis, as healthy spontaneously breathing individuals have the ability to increase their alveolar ventilation. The most common causes of respiratory acidosis are listed in Table 23-3. Respiratory acidosis can be further classified as acute or chronic based on the presence and extent of renal compensation (see below).
Table 23-3 Causes of Respiratory Acidosis
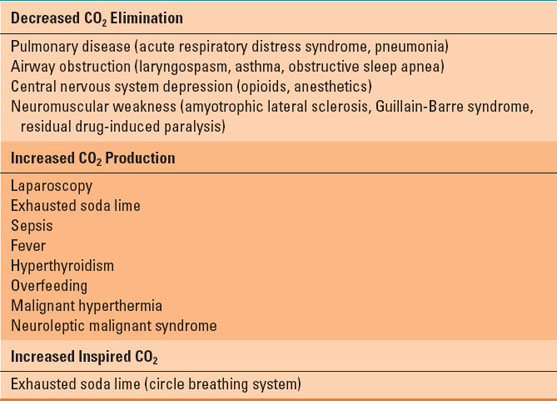
Treatment of respiratory acidosis relies on identification of the underlying cause. The most common interventions include supporting increased alveolar ventilation by institution of noninvasive or invasive mechanical ventilation or reversal of respiratory depressant medications. Avoiding high carbohydrate foods and providing sedative medications will decrease metabolic CO2 production. Sodium bicarbonate is not recommended, as it offers no proven benefit and can worsen the hypercapnea by producing more CO2 (Eq. 23-3).
E. Respiratory Alkalosis
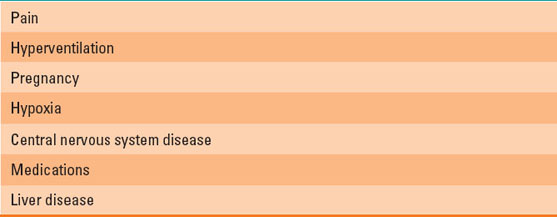
Respiratory alkalosis is defined as an arterial pH >7.45 and PCO2 <36 mm Hg. Because arterial partial pressure of CO2 (PaCO2) is inversely proportional to alveolar ventilation, respiratory alkalosis results from low PaCO2 due to inappropriate alveolar hyperventilation. Equation 23-3 demonstrates that a decrease in CO2 results in fewer H+ ions and therefore an increase in pH. The most common causes of respiratory alkalosis are listed in Table 23-4. Treatment is to identify the underlying cause and provide appropriate treatment.
Table 23-4 Causes of Respiratory Alkalosis
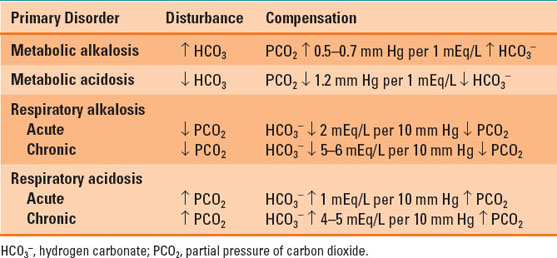
F. Physiologic Compensation of Acid–Base Disorders
Primary metabolic disorders lead to respiratory compensation and vice versa. Respiratory compensation is quite swift. Rapid increases in alveolar ventilation can normalize pH in a matter of minutes. Conversely, metabolic compensation for respiratory disorders takes hours to days, as it requires the kidneys to alter plasma HCO3− levels. Most compensatory responses are quite effective, although respiratory compensation for metabolic alkalosis requires alveolar hypoventilation to increase PCO2, but it is limited to a maximum of 75% due to the resulting hypoxemia that occurs. A list of the normal compensatory responses expected for the acid–base disturbances is provided in Table 23-5.
Table 23-5 Physiologic Compensation for Acid–Base Disturbances
II. Practical Approach to Acid–Base Interpretation
Arterial blood gas (ABG) analysis is primarily used to assess adequacy of gas exchange and oxygen delivery. It is the most frequently ordered test in anesthetized and critically ill patients to guide ventilation and treatment, and it provides data including pH, arterial partial pressure of oxygen (PaO2), PaCO2, and HCO3−. Analysis of these values can be used to determine whether an acid–base disturbance exists, what the disturbance is, and its possible etiologies. Thus, the understanding and ability to quickly analyze ABG data are crucial for every anesthesiologist.
A simple acid–base disorder, typically one of the metabolic or respiratory disturbances occurring in isolation, is the most common clinical presentation. However, critically ill patients can have multiple acid–base disorders. Physiologic compensation is never complete, thus the pH will never be completely normal in a simple acid–base disorder. However, in the presence of multiple acid–base disturbances, the pH can normalize or reach life-threatening extremes.
Arterial blood gas analysis is best approached systematically to ensure rapid and precise interpretation. One common, step-wise method is based on the Henderson-Hasselbalch equation and analyzes the pH, PaCO2, HCO3−, AG, and presence of compensation (Table 23-6).
Table 23-6 Step-wise Approach to Arterial Blood Gas Analysis
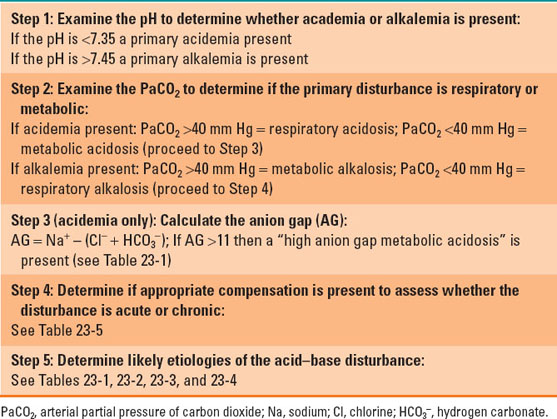
A typical perioperative clinical scenario illustrating the application of this step-wise method is shown below.
Clinical Scenario: Arterial Blood Gas Interpretation
A 52-year-old morbidly obese diabetic female presents to the operating room for emergent debridement of a necrotizing soft tissue infection of her left foot, with the following preoperative ABG (room air):
ABG: pH 7.10, PaCO2 28 mm Hg, PaO2 88 mm Hg, HCO3− 11 mEq/L, Na+ 136 mEq/L, K+ 5.5 mEq/L, Cl− 99 mEq/L, lactate 14 mmol/L
Step 1: because the pH is <7.35, acidemia is present
Step 2: because the PaCO2 is <40, a primary metabolic acidosis is present
Step 3: because the anion gap is [136 – (99 + 11)] 26, a “high anion gap” metabolic acidosis is present
Step 4: because the PaCO2 is appropriately decreased (HCO3− is 13 mEq/L below the normal of 24 mEq/L; 13 × 1.2 mm Hg = 15.6 mm Hg; 28 + 15.6 = 43.6 mm Hg), a compensated metabolic acidosis is present
Step 5: The patient has a severe soft tissue infection and systemic sepsis, with impaired tissue oxygen use resulting in anaerobic metabolism and accumulation of lactic acid, leading to a compensated, high anion gap, metabolic acidosis.
III. Physiology of Fluid Management
The kidneys play several key roles in maintaining homeostasis in the human body. In addition to maintaining normal acid–base status, the kidneys must regulate total body water and solute because daily intake of each is variable. Improper regulation can result in too little body water (cellular dehydration) or too much (tissue edema). Similar regulation occurs when patients have no oral intake and instead are receiving intravenous fluid (i.e., water with dissolved solute). A thorough knowledge of how administered fluids are distributed among the various body compartments, as well as their individual components, is essential. Intravenous fluid should be considered like any other pharmaceutics insofar as there are specific indications and clinical contexts that determine proper fluid therapy. For example, patients residing in the intensive care unit or presenting to the operating room may have low extracellular fluid volume, cellular dehydration, or both as a result of major trauma, hemorrhage, prolonged fasting or malnutrition, or protracted vomiting or diarrhea. Both the choice of fluid composition and its infusion rate are dictated accordingly, in this case, most likely high-volume resuscitation with isotonic crystalloid. The anesthesiologist must take into account such clinical contexts and pathophysiologies when tailoring fluid management for a given patient.
 VIDEO 23-1
VIDEO 23-1
Fluid Warmers
A. Body Fluid Compartments
Total body water (TBW) is estimated to comprise 60% and 50% of the lean body mass of adult males and females, respectively. TBW is distributed throughout various body compartments with roughly 66% making up the intracellular fluid (ICF) and 33% making up the extracellular fluid (ECF). ECF is further divided into interstitial fluid and plasma, which account for approximately 24% and 8% of the TBW, respectively (Fig. 23-2). Thus, for an adult male weighing 70 kg, the TBW is estimated to be 42 L. Of this amount, only 3.5 L is plasma, with the remainder of the circulating blood volume being red blood cells (see also Chapter 3).
 VIDEO 23-2
VIDEO 23-2
Fluid Compartments
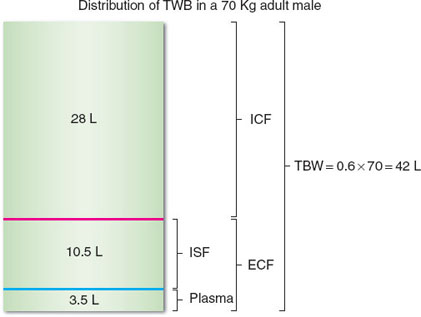
FIGURE 23-2 The approximate distribution of total body water (TBW) in the various body compartments is shown for a 70-kg adult male. ICF, intracellular fluid; ECF, extracellular fluid; ISF, interstitial fluid.
B. Regulation of Extracellular Fluid Volume
The control of ECF concentration and volume is important for cellular function, transfer of molecules between ICF and ECF, and maintenance of circulating blood volume. Normal serum osmolarity is 285 to 295 mOsm/L and is calculated from measured concentrations of sodium, glucose, and urea (blood urea nitrogen [BUN]) as follows:
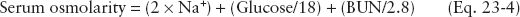

Osmolarity is the number of solute particles per liter of solution, whereas osmolality is the number of particles per kilogram of solvent. Both terms describe the solute concentration of a solution, and due to the negligible difference in their values, are often used interchangeably.
It should be noted, however, that a difference exists between osmolarity (the concentration of particles dissolved per unit of serum volume) and tonicity (the effective osmolarity that can exert an osmotic force across a membrane). Tonic molecules (e.g., Na+) are considered “effective osmoles” because they do not move freely across a membrane. They are able to cause water movement down a concentration gradient. In contrast, because urea freely diffuses across biologic membranes and distributes itself throughout the total body water, it is not tonic. Thus, under conditions of relative normoglycemia, the major contributor to serum osmolarity and tonicity is the Na+ concentration.
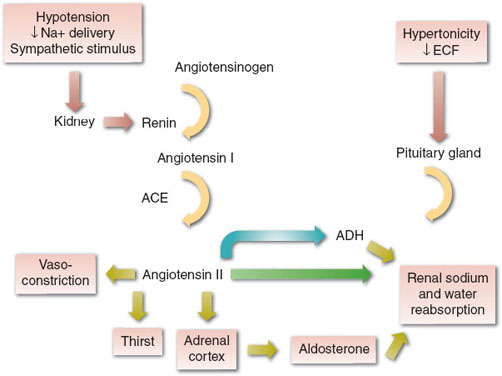
The concentration and volume of the ECF are maintained by both thirst and the hormonal actions of the renin-angiotensin-aldosterone system (RAAS) and antidiuretic hormone (ADH) on the kidneys, which alter the amount of Na+ and water excreted in urine. ADH is released (i.e., nontonic release) from the posterior pituitary in response to small changes (2% to 3%) in serum tonicity or >10% decreases in effective circulating volume. The RAAS is activated by hypotension, the sympathetic nervous system, and decreased Na+ delivery to the kidneys (Fig. 23-3). The end result is increased thirst (increased water intake), increased renal retention of Na+ and water, and vasoconstriction, all of which act to maintain effective circulating volume and perfusion of vital organs.