Thyroid hormone is a nonsteroid hormone, but behaves more like a steroid hormone.
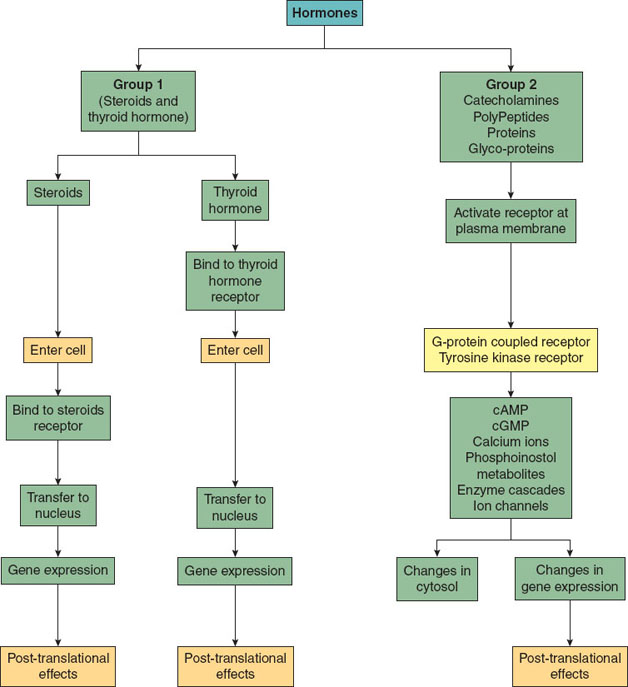
Figure 18-1 Integrated physiology of steroid and nonsteroid hormones.
Nonsteroid hormones include catecholamines, peptides, proteins, or glycoproteins. They are hydrophilic and thus unable to cross the cell membrane and require specific cell membrane receptors to exert their effect. These hormones are typically not bound to plasma proteins, have fast onset of actions (minutes), have shorter half-lives (minutes), and are metabolized quickly. Some hormones are secreted continuously, whereas others are secreted in a pulsatile manner (cortisol).
II. Hypothalamus–Pituitary Complex
In conjunction with the hypothalamus, the pituitary gland is considered a master endocrine gland. Input from various regions of the brain is relayed to specific nuclei in the hypothalamus, which secretes specific releasing factors or hormones that regulate pituitary function (4). The pituitary gland is composed of two parts: the anterior and posterior pituitary.
A. Anterior Pituitary
The anterior pituitary is responsible for producing six hormones that subsequently affect thyroid, adrenal cortex, gonads, and mammary glands. The production and release of anterior pituitary hormones is controlled by releasing hormones (e.g., thyroid-stimulating releasing hormone), which are produced by the hypothalamus. The anterior pituitary hormones are then released into the systemic circulation and exert their effects on their target organs (Fig. 18-2).
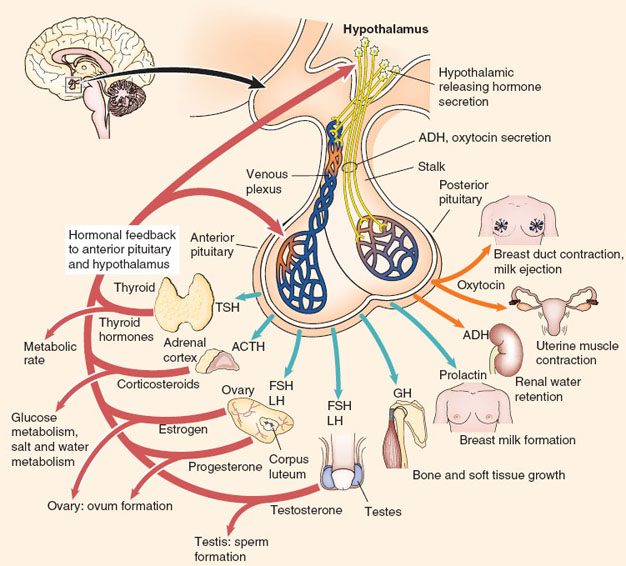
Figure 18-2 Hypothalamus, pituitary gland, and endocrine target organs. (This chapter will focus on hypothalamus, pituitary gland, and the target organs thyroid gland, adrenal gland, parathyroid gland, and the pancreas. For additional information regarding other target endocrine organs, the reader is referred to reference 4.) (From Turbow SD, Patterson BC. Hypothalamic and pituitary disorders. In: Felner EI, Umpierrez GF, eds. Endocrine Pathophysiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014:17, with permission.)
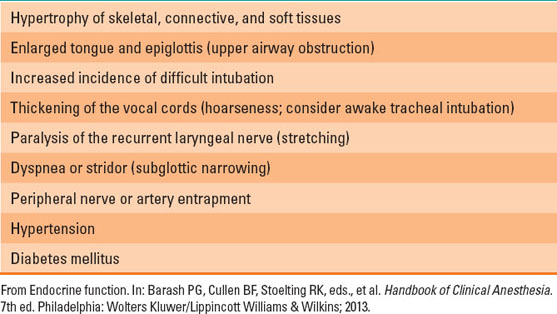
In addition, excessive secretion of growth hormone (GH), usually from a pituitary adenoma, results in acromegaly. Acromegaly occurs after excess GH is produced before epiphyseal closure. In the setting of excess GH, gigantism occurs after epiphyseal closure. Acromegaly is seen much more frequently (Table 18-1).
 VIDEO 18-1
VIDEO 18-1
Pituitary Tumors
Table 18-1 Anesthetic Problems Associated with Acromegaly
B. Posterior Pituitary
Posterior pituitary gland is an extension of the hypothalamus. It produces two hormones: oxytocin and vasopressin (antidiuretic hormone, ADH) (Fig. 18-2) (1).
Diabetes Insipidus
Deficiency of vasopressin (central diabetes insipidus [DI]) or resistance to its effect (nephrogenic DI) causes an inability to absorb water in the renal tubules and collecting ducts (2,3). In neurogenic diabetes, desmopressin leads to concentration of urine. This is not seen in nephrogenic DI. The patient produces liters of dilute urine per day. DI can develop acutely after intracranial surgery, head trauma, intracranial tumors, and infections. If the water loss is not supplemented, either by increased water intake or exogenous supplementation, severe dehydration, hyperosmolality, hypernatremia, cardiovascular collapse, stupor, and coma can result. Central DI can be managed by administration of the vasopressin analogue desmopressin. Perioperative considerations are detailed in Table 18-2.

Certain medications cause nephrogenic diabetes insipidus (e.g., lithium).
Table 18-2 Diabetes Insipidus: Anesthesia Implications

Syndrome of Inappropriate Antidiuretic Hormone Secretion
Excess vasopressin (or inappropriate vasopressin) secretion is a ubiquitous response after trauma and surgery and leads to excess absorption of water by the kidneys. Increased water absorption causes dilution of serum sodium (hyponatremia), decreased serum osmolality, and concentrated urine. In certain pathologic states (i.e., congestive heart failure or cirrhosis), activation of the renin–angiotensin system (2) (see Fig. 5-7 in Chapter 5) can lead to excess vasopressin secretion and cause syndrome of inappropriate antidiuretic hormone secretion (SIADH). Hyponatremia in the setting of concentrated urine strongly suggests SIADH. Typical management is free water restriction, diuretics, and control of any precipitating condition. Severe hyponatremia, levels <115 to 120 mEq/L, is a medical emergency. It causes mental status changes and may require hypertonic saline for correction. Perioperative considerations are detailed in Table 18-3.

The thyroid gland secretes three hormones: thyroxine (T4), triiodothyronine (T3), and calcitonin (involved in calcium homeostasis).
Table 18-3 Syndrome of Inappropriate Antidiuretic Hormone Secretion: Anesthesia Implications

III. Thyroid Gland
The thyroid gland is one of the largest endocrine glands. Thyroxine (T4) and triiodothyronine (T3) are under tight control of thyroid-stimulating hormone (TSH) from the pituitary gland (Fig. 18-3) (3).
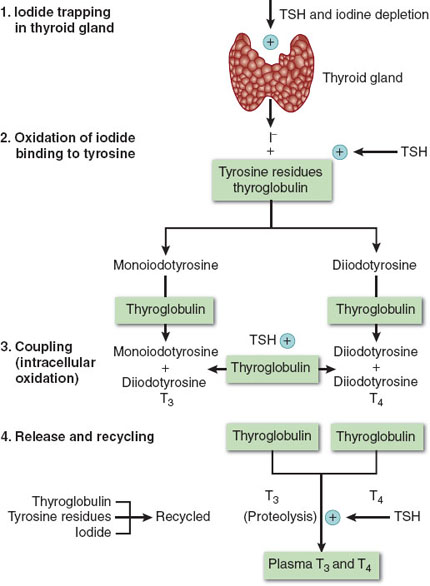
Figure 18-3 Thyroid hormone biosynthesis consists of four stages: (1) organification, (2) binding, (3) coupling, and (4) release. TSH, thyroid-stimulating hormone; T3, triiodothyronine; T4, thyroxine. (From Schwartz JJ, Akhtar S, Rosenbaum SH. Endocrine function. In: Barash PG, Cullen BF, Stoelting RK, et al., eds. Clinical Anesthesia. 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2013:1327, with permission.)
A. Thyroid Hormone Metabolism
Tyrosine and iodine are needed to form T4 and T3 (Fig. 18-3). Absorbed iodine is converted to iodide and transported and concentrated in thyrocytes. Tyrosine, which is attached to thyroglobulin, is then iodinated by a complex process to yield T3 and T4 (Fig. 18-3). T4 is converted in the peripheral tissues to T3. T4 feeds back to the pituitary and hypothalamus and decreases the secretion of TSH, which then leads to a decrease in T4. T4 and T3 are lipophilic and are 99.8% bound to albumin, thyroxine-binding globulin, and prealbumin. It is the free hormone that exerts a biologic effect. T4 and T3 are metabolized in the liver, kidney, and many other tissues. Glucocorticoids, dopamine, somatostatin, and stress decrease TSH secretion.

Twenty times more T4 is produced by the thyroid gland than T3. However, T3 is the more active form and produces the preponderance of clinical effects.
B. Physiologic Effects of Thyroid Hormone
T3 and T4 increase oxygen consumption of target organs. Thyroid hormones increase carbohydrate, fat, and protein metabolism and are essential for normal growth. They increase cardiac output by increasing heart rate and myocardial contraction and enhance the effect of circulatory catecholamines. Thyroid hormones have marked effects on brain and enhance catecholamine effect on the reticular activating system.
C. Tests of Thyroid Function
Abnormalities in thyroid function are seen in both thyroid and nonthyroid diseases (Table 18-4). The initial step is to measure TSH and free T4 (fT4) (3). Extremely low TSH in the setting of high fT4 suggests hyperthyroidism, while high TSH and low fT4 suggest hypothyroidism.
Table 18-4 Tests of Thyroid Function
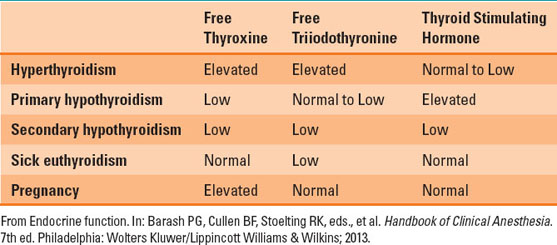
D. Hyperthyroidism
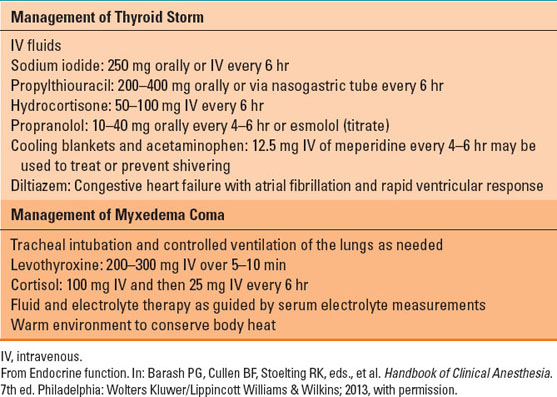
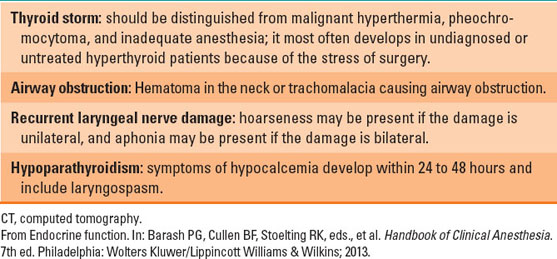
Hyperthyroidism is characterized by nervousness, weight loss, hyperphagia, heat intolerance, sweating, tachycardia, and increase in pulse pressure (because of vasodilation). Patients who present with uncontrolled hyperthyroid symptoms should be managed medically before elective surgery. The basic principle of medical management of hyperthyroidism is decreasing thyroid hormone production and blunting the hyperadrenergic symptoms. Beta-blockers decrease adrenergic symptoms (tachycardia, increased cardiac output), while propylthiouracil and methimazole compete with tyrosine for iodide and thus block T3 and T4 production. In acute situations, exogenous iodine can be administered, which depresses thyroid hormone production. After adequate control of hyperthyroid symptoms, surgical excision of the thyroid gland can be performed. Radioactive iodine ablates the thyroid gland and leads to a progressive loss of function. Loss of thyroid function is replaced with exogenous levothyroxine. Acute thyroid storm is a medical emergency. Patients can present with or develop it intraoperatively. Management of thyrotoxicosis is detailed in Table 18-5.
Table 18-5 Perioperative Thyroid Emergencies (Thyrotoxicosis and Myxedema Coma)
E. Hypothyroidism
Patients with hypothyroidism present with peripheral vasoconstriction, poor mentation, cold intolerance, and weight gain. Hypothyroidism is treated with exogenous levothyroxine. Patients with severe hypothyroidism should be treated prior to elective procedures. In this situation, T3 can be used, which has a faster onset of action. These patients are exquisitely sensitive to sedative medications and can quickly develop cardiorespiratory collapse. Management of myxedema coma requires inotropic agents, administration of intravenous T4 or T3, fluids, hydrocortisone, and respiratory support (Table 18-5).
F. Anesthetic Priorities in Thyroid Surgery
General anesthesia with an endotracheal tube is used for thyroid surgery (2,3). Anesthesiologists may encounter unexpected difficult airway in 5% to 8% of cases. Retrosternal thyroid behaves as an anterior mediastinal mass, and special consideration should be kept in mind (see Chapter 34). Recurrent laryngeal nerve injury is a distinct possibility, leading to airway compromise after surgery (see Chapter 20) (Table 18-6). Tracheal compression due to hematoma or tracheomalacia (after excision of a large thyroid) is an emergency (see Chapter 20). Hypocalcemia due to hypoparathyroidism can develop within 24 to 96 hours after surgery.
Table 18-6 Complications of Thyroid Surgery
VI. Adrenal Gland
A. Adrenal Cortex
The adrenal gland is composed of two parts: the outer cortex and the inner medulla. The cortex is divided into three zones—the zona glomerulosa, zona fasciculata, and zona reticularis—which produce mineralocorticoids, glucocorticoids, and androgens, respectively (Fig. 18-4) (4–6). The precursor of all steroid hormones is cholesterol, which is converted to pregnenolone in the mitochondria and transported to the cytoplasm and converted by various enzymes into specific steroid hormones.
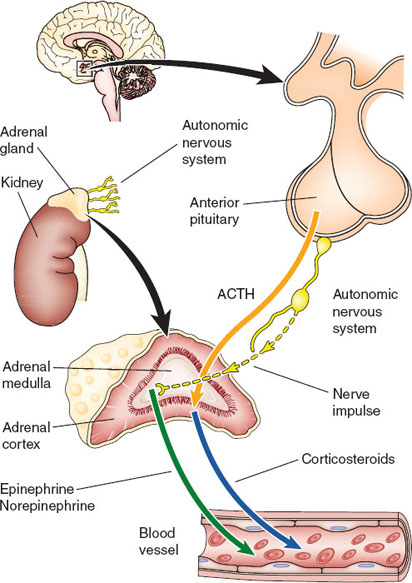
Figure 18-4 The hypothalamic–pituitary axis controls many of the functions of the normal adrenal gland. The cortex is responsive to corticotropin, while the medulla is under control of the autonomic nervous system. (From Hammel JA, Umpierrez GE. Adrenal gland disorders. In: Felner EI, Umpierrez GF, eds. Endocrine Pathophysiology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2014:480.)
Physiologic Effects of Glucocorticoids
Glucocorticoid production and secretion is controlled by corticotropin from the pituitary gland. Glucocorticoids increase protein catabolism, glycogenolysis, and gluconeogenesis and have an anti-inflammatory and anti-insulin effect. They are required for glucagon and catecholamines to have their metabolic effects, normal vascular reactivity, neurologic function, and water excretion. Glucocorticoids also decrease eosinophils and basophils but increase neutrophils, platelets, and red blood cells (3).
Glucocorticoid Excess (Cushing Syndrome)
Glucocorticoid excess leads to Cushing syndrome. This excess can be due to either an increase in corticotropin (corticotropin dependent) or increased production by the adrenal gland (corticotropin independent). Corticotropin-dependent production, also called Cushing disease
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







