Peter Slinger
The most common indication for thoracic surgery is malignancy (1,2,3). Despite this, a wide variety of pathologies and procedures are commonly encountered when providing anesthetics for patients undergoing thoracic surgery. As a result, there are a number of important preoperative, intraoperative, and postoperative anesthetic considerations for thoracic surgery.
I. Preoperative Assessment
Respiratory and cardiac complications are the major cause of perioperative morbidity and mortality in the thoracic surgical population. Thus, the preoperative evaluation of these patients focuses on an assessment of respiratory function and the cardiopulmonary interaction. All pulmonary resection patients should have preoperative spirometry to determine postoperative preservation of respiratory function, which has been shown to be proportional to the remaining number of lung subsegments (right upper, middle, and lower lobes = 6,4,12 subsegments, respectively; left upper and lower lobes = 10 subsegments each, for a total of 42 subsegments). The principles discussed in the sections that follow also apply to thoracic surgical patients who are not having lung resections (1,2).
A. Lung Mechanical Function
A valid single test for postthoracotomy respiratory complications is the predicted postoperative (ppo) forced expiratory volume in 1 second (ppoFEV1%), which is calculated as:
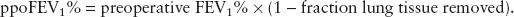
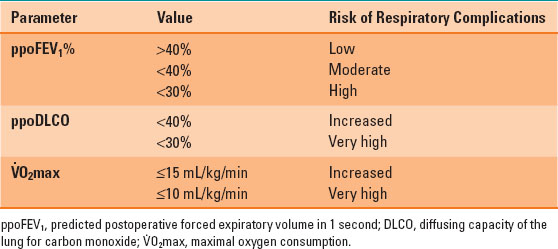
For example, a patient with a preoperative FEV1 of 60% having a right upper lobectomy (6 of 42 lung subsegments) would be expected to have a ppoFEV1% = 60% × [1 – (6/42)] = 51%. Patients with a ppoFEV1 >40% are at low risk for postresection respiratory complications, <40% at moderate risk, and <30% are at high risk (1,2).

Patients with a predicted postoperative FEV1 of >40% are at low risk for postoperative respiratory complications.
B. Lung Parenchymal Function
The most useful test of gas exchange is the diffusing capacity of the lung for carbon monoxide (DLCO). The preoperative DLCO can be used to calculate a ppo value using the same calculation as for the FEV1, with similar risk categories: increased risk <40% and high risk <30% (1,2,3).
C. Cardiopulmonary Interaction
The most important assessment of respiratory function is an assessment of the cardiopulmonary reserve, and the maximal oxygen consumption ( O2max) is the most useful predictor of outcome. The risk of morbidity and mortality is increased if the preoperative V.O2max is ≤15 mL/kg/min and very high if it is ≤10 mL/kg/min. In ambulatory patients, the V.O2max can be estimated from the distance in meters that a patient can walk in 6 minutes (6-minute walk test [6MWT]) divided by 30 (i.e., 6MWT of 450 m:estimated V.O2max = 450/30 = 15 mL/kg/min). The ability to climb five flights of stairs correlates with a V.O2max >20 mL/kg/min, and two flights corresponds to a V.O2max of 12 mL/kg/min (1,2,3) (Table 34-1).
O2max) is the most useful predictor of outcome. The risk of morbidity and mortality is increased if the preoperative V.O2max is ≤15 mL/kg/min and very high if it is ≤10 mL/kg/min. In ambulatory patients, the V.O2max can be estimated from the distance in meters that a patient can walk in 6 minutes (6-minute walk test [6MWT]) divided by 30 (i.e., 6MWT of 450 m:estimated V.O2max = 450/30 = 15 mL/kg/min). The ability to climb five flights of stairs correlates with a V.O2max >20 mL/kg/min, and two flights corresponds to a V.O2max of 12 mL/kg/min (1,2,3) (Table 34-1).
Table 34-1 Summary of Important Values in the Preoperative Respiratory Assessment
D. Cardiac Investigations
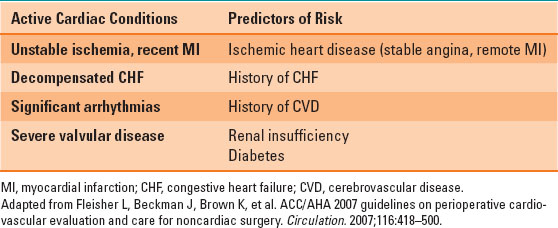
Thoracic surgery is considered “intermediate risk” for cardiac complications, such as myocardial infarction and arrhythmias, according to the American College of Cardiology and the American Heart Association (ACC/AHA) guidelines for the preoperative assessment of cardiac patients undergoing noncardiac surgery. Patients with cardiac conditions or risk factors (Table 34-2) should be investigated according to the ACC/AHA guidelines: noninvasive stress testing or cardiac catheterization should be performed in these patients if they have poor or unknown functional capacity, three or more clinical risk factors, and, most important, if the results of the testing will change management (1,2,4).
Table 34-2 Cardiac Conditions and Risk Factors
E. Common Pathologies and Comorbidities
Malignancies
The majority of patients presenting for thoracic surgery will have a malignancy, including lung cancers, pleural and mediastinal tumors, and esophageal cancer. These patients should be assessed for the “4-M’s” associated with malignancy: Mass effects (obstructive pneumonia, superior vena cava [SVC] syndrome, etc.), Metabolic abnormalities (hypercalcemia, Lambert-Eaton syndrome, etc.), Metastases (brain, bone, liver, and adrenal), and Medications (adjuvant chemotherapy and radiation) (1,2).

The “4-M’s” associated with malignancy are: Mass effect, Metabolic abnormalities, Metastases, and Medications.
Chronic Obstructive Pulmonary Disease
This is the most common concurrent illness in the thoracic surgical population. Patients should be free of exacerbation before elective surgery, and may have fewer postoperative pulmonary complications when intensive chest physiotherapy is initiated preoperatively. Pulmonary complications are also decreased in thoracic surgical patients who cease smoking for more than four weeks before surgery. Patients with chronic obstructive pulmonary disease and limited or unknown exercise tolerance may benefit from an arterial blood gas (ABG) preoperatively, if the results prove helpful in weaning mechanical ventilation at the conclusion of surgery. Other considerations in chronic obstructive pulmonary disease patients include the presence of bullous disease, pulmonary hypertension with right heart dysfunction, and the risk of dynamic hyperinflation due to gas trapping (1,2,3).
II. Intraoperative Management
A. Monitoring
Standard anesthetic monitoring is used in all thoracic surgery cases. An invasive arterial line is placed for the majority of surgeries. It is useful to measure baseline preoperative ABGs for intraoperative comparison during one-lung ventilation (OLV), detection of sudden blood pressure changes, and postoperative weaning of mechanical ventilation. A central venous line may be required in some cases for vascular access or for infusion of vasoactive medications. The central venous pressure (CVP) can be a useful intraoperative and postoperative monitor, particularly for cases where fluid management is critical, such as pneumonectomies and esophagectomies. Fluid management for all thoracic procedures should follow either a restricted or a goal-directed protocol. However, recently, concerns about acute kidney injury have called into question the strategy of fluid restriction in thoracic surgery (3). No fluids are given for theoretical “third-space” losses. Colloids have not been proven to improve outcome and add considerable expense. Spirometry is particularly useful to monitor breath-by-breath inspired and expired tidal volumes during OLV and may alert the clinician to possible loss of lung isolation, air leaks, and the development of hyperinflation (1,3).
B. Physiology of One-Lung Ventilation
In the majority of thoracic surgery cases, patients transition from being upright, awake, and spontaneously breathing to supine, asleep, and paralyzed. They are then moved from the supine position to the lateral position. Finally, OLV is initiated and their chest is opened. Changes in ventilation and perfusion accompany each of these circumstances.
First, functional residual capacity (FRC) is the main factor determining oxygen reserve in patients when they become apneic. Patients will experience a decrease in FRC when in the supine position compared with the upright position. This change will be magnified by the induction of anesthesia and administration of muscle relaxants. When upright, the majority of ventilation and perfusion reach the gravity-dependent portions of the lungs (i.e., the bases). With the induction of anesthesia, most of the ventilation now enters the nondependent portions of the lung, increasing ventilation-perfusion (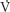 /
/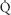 ) mismatch.
) mismatch.
Second, in the lateral position, the dependent lung receives more perfusion compared with the nondependent lung. However, the dependent hemidiaphragm is pushed into the thoracic cavity by the abdominal contents, further decreasing FRC and worsening V./Q. mismatch.
Third, when the chest is opened, the compliance of the nondependent lung improves relative to the dependent lung, and it is preferentially ventilated, further increasing the V./Q. mismatch. However, when OLV is initiated in the dependent lung, it receives the majority of both perfusion and ventilation. There will still be some cardiac output shunted through the collapsed, nondependent lung, but V./Q. matching may be improved by hypoxic pulmonary vasoconstriction (HPV) in the nonventilated, nondependent lung (Fig. 34-1). HPV can be inhibited by many factors, such as extremes of pulmonary artery pressures, hypocapnia, vasodilators, and inhalational agents (1,3).
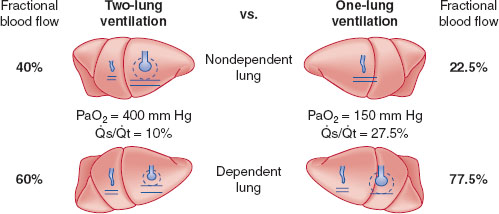
FIGURE 34-1 Schematic representation of two-lung ventilation versus one-lung ventilation (OLV). Typical values for fractional blood flow to the nondependent and dependent lungs, as well as PaO2 and 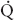 s/
s/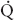 t for the two conditions, are shown. The
t for the two conditions, are shown. The 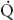 s/
s/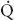 t during two-lung ventilation is assumed to be distributed equally between the two lungs (5% to each lung). The essential difference between two-lung ventilation and OLV is that, during OLV, the nonventilated lung has some blood flow and therefore an obligatory shunt, which is not present during two-lung ventilation. The 35% of total flow perfusing the nondependent lung, which was not shunt flow, was assumed to be able to reduce its blood flow by 50% by hypoxic pulmonary vasoconstriction. The increase in
t during two-lung ventilation is assumed to be distributed equally between the two lungs (5% to each lung). The essential difference between two-lung ventilation and OLV is that, during OLV, the nonventilated lung has some blood flow and therefore an obligatory shunt, which is not present during two-lung ventilation. The 35% of total flow perfusing the nondependent lung, which was not shunt flow, was assumed to be able to reduce its blood flow by 50% by hypoxic pulmonary vasoconstriction. The increase in 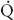 s/
s/ t from two-lung to OLV is assumed to be due solely to the increase in blood flow through the nonventilated, nondependent lung during OLV. (From Eisenkraft JB, Cohen E, Neustein SM. Anesthesia for thoracic surgery. In: Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:1041.)
t from two-lung to OLV is assumed to be due solely to the increase in blood flow through the nonventilated, nondependent lung during OLV. (From Eisenkraft JB, Cohen E, Neustein SM. Anesthesia for thoracic surgery. In: Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:1041.)
C. Indications for One-Lung Ventilation
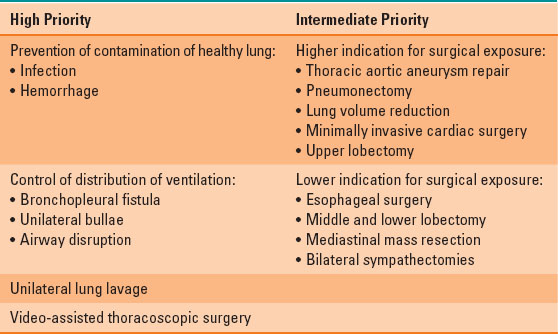
High and intermediate priorities for OLV are listed in Table 34-3. The highest priorities include prevention of contamination of the healthy lung by infection or hemorrhage; control of distribution of ventilation in bronchopleural fistula, unilateral bullae, or airway disruption; unilateral lung lavage; and video-assisted thoracoscopic surgery (VATS). Intermediate priorities for OLV include surgical exposure in thoracic aortic aneurysm repair, pneumonectomy, lung volume reduction, minimally invasive cardiac surgery, and upper lobectomy. Lower indications for OLV include surgical exposure in esophageal surgery, middle and lower lobectomy, mediastinal mass resection, and bilateral sympathectomies (1,3).
Table 34-3 Indications for Lung Isolation
D. Methods of Lung Isolation
Lung isolation can be achieved with the use of a double-lumen tube (DLT), bronchial blocker, or endobronchial intubation with a regular single-lumen tube (SLT) or specialized endobronchial tube. An SLT is rarely used in an endobronchial fashion in adults except in emergent scenarios, as bronchoscopy, suction, or continuous positive airway pressure (CPAP) cannot be applied to the collapsed lung.
 VIDEO 34-1
VIDEO 34-1
Double Lumen Tube Description
DLTs are the most commonly used method to achieve lung isolation and OLV (Fig. 34-2). They are available in both left- and right-sided conformations, with the left-sided DLT being the most widely used. Advantages of the DLT include the ability to isolate either lung; apply suction, CPAP, or oxygen insufflation down either lumen; and to perform bronchoscopy down either lumen. The DLT is less likely to dislodge than other methods of lung separation, which makes it the preferred method of isolation in cases of infection or hemorrhage. Disadvantages of the DLT include the fact that it is more challenging to place in a difficult airway, and it will usually need to be exchanged for an SLT if a patient is to remain intubated postoperatively.
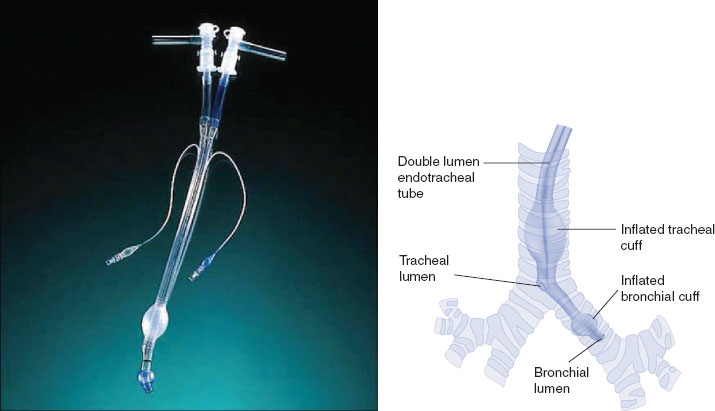
FIGURE 34-2 A left-sided Robertshaw type double-lumen tube constructed from polyvinyl chloride (left). When properly positioned (right), the distal “bronchial lumen” is placed in the left mainstem bronchus proximal to the left upper lobe orifice, with the “bronchial cuff” inflated just distal to the carina in the left mainstem bronchus. The proximal “tracheal lumen” is positioned above the carina, with the “tracheal cuff” inflated in the mid-trachea. Proper positioning allows for the options of one-lung ventilation on either side (with contralateral lung deflation), as well as two-lung ventilation. (From Eisenkraft JB, Cohen E, Neustein SM. Anesthesia for thoracic surgery. In: Barash PG, Cullen BF, Stoelting RK, et al. Clinical Anesthesia, 7th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:1043.)
A bronchial blocker
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








