Levels of evidence: Ia, evidence from meta-analysis; III, evidence from well-designed, nonexperimental descriptive studies such as comparative studies or case reports; IV, expert opinion.
Table modified from Neal JM. Ultrasound-guided regional anesthesia and patient safety: an evidence-based analysis. Reg Anesth Pain Med 2010;35:S59–S67, with permission from the American Society of Regional Anesthesia and Pain Medicine.
 DEFINITION
DEFINITION
This chapter defines permanent nerve injury as a neural deficit that remains 1 year after block placement, a definition that is generally agreed upon by neurologists. Injuries <1 year in duration are referred to as PONS, which describes early and delayed neural symptoms that usually are transient. Although early after nerve injury PONS can serve as a surrogate marker for permanent nerve injury, the rate of conversion from PONS to permanent injury is unknown. LAST refers to any central nervous system (CNS) or cardiac manifestation of local anesthetic toxicity, but this chapter focuses only on the most serious complications—seizure and/or cardiac arrest.
 POSTOPERATIVE NEUROLOGICAL SYMPTOMS
POSTOPERATIVE NEUROLOGICAL SYMPTOMS
Scope
The incidence of regional anesthesia-associated peripheral nerve injury is difficult to define and is significantly influenced by the time elapsed since block placement. Because permanent block-related injury is extremely rare, most studies report surrogate PONS metrics, for example, persistent paresthesia or transient sensory and/or motor dysfunction. In the first 24 hours after peripheral nerve block, PONS may be present in up to 19% of patients. Over the ensuing weeks and months, these symptoms typically resolve, with well over 99% no longer present at 1 year.2,6 The large French surveillance studies of the late 1990s/early 2000s reported that neurologic injury was still present at 6 months in 1.47 to 1.98 per 10,000 patients (95% confidence interval [95% CI] 0.5–4.8/10,000).8 Data are much less robust for defining the incidence of permanent injury. In a review of neurologic complications published between 1995 and 2005, only one permanent injury was reported in 65,092 blocks.9
Pathophysiology
The pathophysiology of peripheral nerve injury is discussed in detail in Chapter 14. Recent research has vastly expanded and indeed changed our understanding of needle-to-nerve proximity and the postulated mechanisms of injury. This section aims to review the pathophysiology of peripheral nerve injury as it relates specifically to nerve localization technique.
The generally accepted mechanism of regional anesthesia-associated peripheral nerve injury posits the concept that needle damage to a nerve’s structural integrity mechanically injures the nerve and/or disrupts the nerve-blood barrier, which in turn allows local anesthetic to contact denuded fascicles and result in neurotoxicity. Prior to ultrasound’s contributions as a powerful research tool for studying nerve injury, most experts presumed that any needle breach of the nerve’s structural integrity was capable of causing injury.10 Despite previous animal studies that clearly demonstrate the perineurium is the most important physical barrier between local anesthetic and nerve axons, much clinical literature fails to differentiate between a needle that penetrates only the epineurium versus one that disrupts the perineurium, thereby gaining access to the nerve fascicles. In these descriptions, needle penetration into a nerve is referred to generically as “intraneural injection” without regard to what component of the nerve’s architecture is breached. With the advent of ultrasound, two observations quickly became apparent. First, PNS- and paresthesia-seeking needles penetrate the epineurium and come to lie within the nerve’s connective tissue much more frequently than clinicians had suspected previously.11 Second, this breach is rarely associated with injury. In animal experiments, histologic and functional injury occurs only when the needle is intentionally (and often with great difficulty) placed within the perineurium to disrupt the fascicular architecture. In summary, peripheral nerve injury theory presumes that needle disruption of the nerve’s intraperineurial architecture is a prelude to at least some forms of neural injury. If this is indeed the case, then nerve localization techniques that avoid potentially harmful needle-to-perineurium contact should reduce the risk of nerve injury.
Within this framework of nerve injury pathophysiology, we can now examine the theoretical contributions of the various nerve localization techniques. Ultrasound-assisted animal and human studies have improved our understanding of PNS-guided needle-to-nerve contact. These studies consistently report that stimulating needles can be extraneural or subepineurial at a wide range of stimulating currents. For instance, an axillary block study reported that paresthesia was only 38% sensitive, and motor response 75% sensitive (≤0.5 mA), for confirming (using ultrasound visualization) needle-to-nerve contact.12 A similar 83% motor block sensitivity (0.2–0.5 mA) was reported for popliteal sciatic nerve block.13 For the supraclavicular approach, a human study documented subepineurial placement of a block needle when the stimulating current was ≤0.2 mA, but could not distinguish extraneural from intraneural needle placement between >0.2 and ≤0.5 mA.14 Animal studies confirm this absence of consistency between electrical current and actual needle position,15 suggesting that PNS is a fallible descriptor of needle-to-nerve relationship (Fig. 17-1). For ultrasound-guided localization techniques, studies show that needle tips can be observed to touch and indent nerves14 or herald subepineurial placement by observing nerve swelling upon injection of local anesthetic14–18 (Fig. 17-2). However, nerve swelling has not been correlated with actual nerve injury,18 suggesting that ultrasound may more accurately depict needle-to-nerve contact as compared with PNS, but that the relationship of needle contact to actual injury remains unknown. Therefore, neither PNS nor UGRA is entirely sensitive or a specific tool for determining needle-to-nerve contact in a manner that predictably recognizes or prevents peripheral nerve injury.
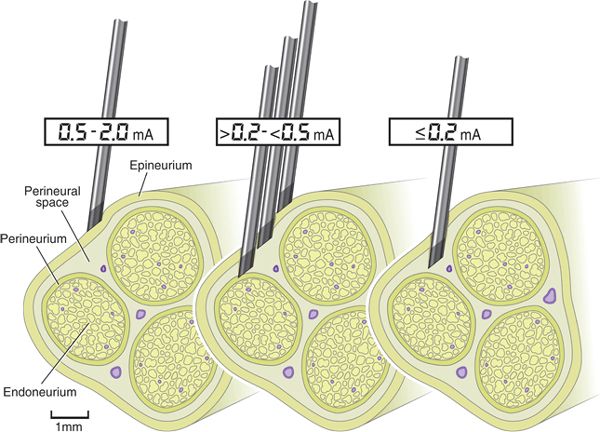
FIGURE 17-1. PNS has limited sensitivity for accurately indicating needle placement within a nerve. At ≤0.2 mA, the needle is reliably intraneural, that is, subepineurial, but may be inside or outside the perineurium. Between 0.2 and ≥0.5 mA, the needle could reside at any location within the nerve or be completely extraneural. Figure created using human supraclavicular nerve block data as reported by Bigeleisen et al.14 and human popliteal sciatic nerve block data as reported by Robards et al.13 (Modified with permission from Anesthesiology and Anesthesia and Analgesia.)
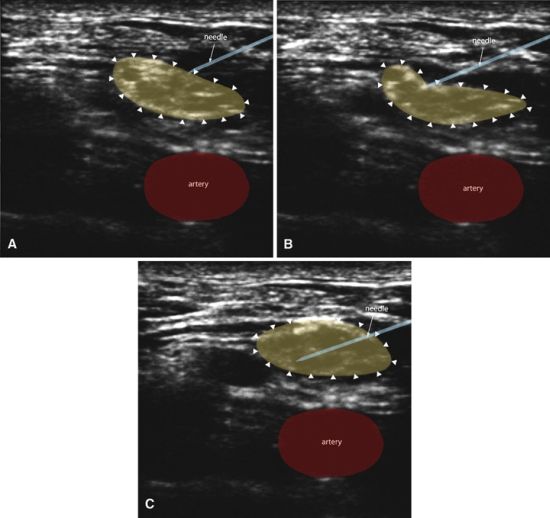
FIGURE 17-2. Ultrasound guidance can reveal needle-to-nerve proximity in several different ways. The needle can be seen to indent the nerve (B) or actually penetrate the nerve (C). If the needle is intraneural, the nerve can be observed to swell during injection of local anesthetic. Figure created using human supraclavicular nerve block sonograms as reported by Bigeleisen et al.14 (Modified with permission from Anesthesiology.)
Effect of Nerve Localization Technique
Although studies regarding PONS as a function of nerve localization technique are limited, several suggest that there is no difference in the incidence of significant injury. A study that compared paresthesia-seeking and PNS techniques for interscalene block found no difference in the incidence of paresthesia (9.3% vs. 10.1%, respectively).19 A study that compared PNS to UGRA for interscalene block found no difference in PONS at 4- to 6-week follow-up (7% vs. 6%, respectively).20 In the Australasian Regional Anaesthesia Collaboration study of over 7,000 nerve blocks,21 the incidence of transient (up to 60 days follow-up) PONS was not statistically different between those patients who underwent regional anesthesia using either PNS or UGRA localization techniques. Although historical comparisons are problematic, it is interesting that the frequency of early and late PONS (4/10,000) is nearly identical to the frequency of nerve injury reported a decade earlier from the French surveillance study, in which nearly all patients underwent PNS-directed block.7,8,22 A slightly smaller quality assurance study23 also found no statistical difference in the incidence of early neurologic symptoms between PNS and UGRA. That UGRA is not completely protective against transient or permanent peripheral nerve injury has been demonstrated by several case reports of injuries despite its use.24–26
In summary, it is unlikely that an adequately powered study to differentiate the effect of nerve localization procedure on late postoperative symptoms, much less permanent injury, will ever be completed27 (Box 17-1). The few studies that use the surrogate PONS markers lend no convincing argument that transient nerve injury is significantly affected by localization technique. To date, there are no studies that focus on those patients most at risk for peripheral nerve injury, such as those with diabetes or chemotherapy-induced neuropathy. These groups may prove to be a better model to study the relative safety effects of various nerve localization techniques.
BOX 17-1 Proving Safety for Rare Events
To “Prove” a 50% reduction in long-term nerve injury from 4/10,000 to 2/10,000
alpha 0.05, beta 0.8
would require
over 70,000 subjects per group
 LOCAL ANESTHETIC SYSTEMIC TOXICITY
LOCAL ANESTHETIC SYSTEMIC TOXICITY
Scope
Similar to nerve injury, the incidence of LAST largely depends on how it is defined. Early markers of local anesthetic vascular uptake, such as CNS excitation and hemodynamic changes, may occur as frequently as 1/1,000 patients.28 However, life-threatening toxicity from seizures or cardiac arrest is exceedingly rare.29 The rate of peripheral nerve block-related seizure reported in the French surveillance studies was 7.5/10,000 patients (95% CI, 3.9–11.2/10,000), and no patient in 21,278 blocks experienced a LAST-associated cardiac arrest.8 Unintentional vascular puncture, as diagnosed by aspiration, hematoma formation, or direct observation with ultrasound, is a surrogate marker for LAST. While vascular puncture is clearly a precursor to more serious complications, there are no data to accurately define how often it is linked to clinically apparent LAST.
Pathophysiology
The pathophysiology of LAST relates to nerve localization technique in one of two ways. First, immediate LAST implies inadequate detection of intravascular injection, which the localization technique should identify ideally prior to injecting significant amounts of local anesthetic. There are no universally recognized signs of blood vessel entry when using PNS. Conversely, UGRA offers the possibility of either avoiding vascular puncture or halting further injection if local anesthetic cannot be observed surrounding the target tissue. Second, UGRA enables the use of smaller volumes, which might temper toxicity by limiting the availability of local anesthetic for tissue uptake.
Effect of Nerve Localization Technique
Evidence that nerve localization technique influences the occurrence of LAST is equivocal. Individual studies and a meta-analysis30 provide strong evidence that the use of ultrasound significantly reduces the incidence of unintentional vascular puncture as compared with PNS techniques. However, it is less clear whether this surrogate outcome equates to a reduction in the true outcomes of seizure and/or cardiac arrest. The Australasian Collaboration found no difference in the incidence of serious LAST events between UGRA and PNS techniques.21 In this study, the 0.98/1,000 incidence of LAST is remarkably similar to the 0.8/1,000 incidence previously reported for PNS.7 The Pittsburgh quality assurance study showed a statistically significant advantage (p = 0.044) to UGRA over PNS with regard to the incidence of seizure, but the absence of an epinephrine test dose could have influenced the incidence of LAST in either group.23
Ultrasound guidance consistently achieves comparable anesthesia using smaller local anesthetic volumes. For adult doses, this reduction in volume is statistically significant, but may still be capable of causing a serious episode of LAST. For instance, ultrasound facilitates the reduction of mean volume from 26 mL using PNS to 15 mL (95% CI, 7–23) during femoral nerve block,31 yet even 15 mL of a potent local anesthetic could be problematic if injected intravascularly, particularly in patients at increased risk for LAST, for example, cardiac disease or extremes of age.32 The ability to anesthetize a target nerve using less local anesthetic may be most useful in pediatrics, where their smaller size places children at increased risk for LAST.32 No studies have directly evaluated LAST in pediatric patients, although as with adults, the risk of unintentional vascular puncture is reduced by the use of ultrasound.33 Importantly, UGRA has been linked to faster absorption and higher plasma concentrations of local anesthetic in children, which suggests that using the lowest volume possible in these populations is not just advantageous, but warranted.34
 HEMIDIAPHRAGMATIC PARESIS
HEMIDIAPHRAGMATIC PARESIS
Scope
When PNS techniques are used for interscalene blocks, the incidence of HDP approaches 100%.35 When local anesthetic is deposited distally along the brachial plexus, the incidence of HDP drops to approximately 50% at the supraclavicular approach36 and nears 0% with the most lateral infraclavicular approaches.2 The incidence of HDP is significantly less with UGRA techniques—13% to 45% for the interscalene approach37,38 and 95% CI 0% to 14% for the supraclavicular approach.39
Pathophysiology
With PNS techniques, local anesthetic conduction block of the phrenic nerve can occur directly as it courses atop the anterior scalene muscle, or from the spread to the C3-C5 nerve roots within the prevertebral fascial compartment.35,40 Both of these mechanisms imply a volume effect, particularly as the local anesthetic is placed distal along the brachial plexus, that is, more distant from the affected nerve roots. Conversely, ultrasound-guided approaches allow for brachial plexus anesthesia with smaller volumes of local anesthetic. Although this likely limits local anesthetic spread within the prevertebral fascial plane, the phrenic nerve nevertheless lies within 2 mm of the C5 nerve root at the level where a block needle is typically directed during an ultrasound-assisted interscalene approach.41
Effect of Nerve Localization Technique
The incidence of HDP is clearly reduced by using UGRA instead of PNS. This finding is likely volume related. In studies of interscalene37,38 and supraclavicular39 brachial plexus blocks, the incidence and severity of HDP is reduced when UGRA is utilized. Some investigators have reported reducing the incidence of HDP associated with the interscalene approach to 45% using 5 mL ropivacaine 0.5% (C5-C6 level)38 or 13% using 10 mL ropivacaine 0.75% (C7 level),37 while other investigators have reported no change in incidence using 10 or 20 mL ropivacaine 0.5% at the cricoid level.42 For the supraclavicular approach, the diaphragm is even less affected and HDP has been reported as 0%, 95% CI 0% to 14% using 20 mL ropivacaine 0.75%,39 or 1%, 95% CI 0.4% to 2.3% using 33 ± 8 mL.43 Despite these impressive results, the practical significance of the reduced incidence of HDP is questionable, since it is never completely eliminated at the interscalene approach. Since HDP is neither completely eliminated nor is it possible to predict individual patient response, we cannot know with certainty when it is safe to administer a low-volume interscalene block to those individuals most at risk for significant HDP, that is, a patient unable to withstand a 30% reduction in pulmonary function.27
 PNEUMOTHORAX
PNEUMOTHORAX
Scope
The precise incidence of pneumothorax is unknown, but is certainly well less than the 0.5% to 6% reported decades ago using the classic approaches to the supraclavicular portion of the brachial plexus.2 Based on projections from recent studies of ultrasound-guided supraclavicular block, the upper limit 95% CI for pneumothorax is 0.5%.27
Effect of Nerve Localization Technique
Patients who undergo above the clavicle and medial infraclavicular approaches to the brachial plexus are at risk for pneumothorax from a needle penetrating the pleura. Although the pleura is easily identifiable using ultrasound, case reports have nevertheless documented pneumothoraces during supraclavicular44 and infraclavicular45 blocks. There are no direct comparisons of UGRA to PNS for this complication.
 UNINTENDED DESTINATIONS OF LOCAL ANESTHETICS
UNINTENDED DESTINATIONS OF LOCAL ANESTHETICS
Scope
Local anesthetic may be deposited within or spread to the neuraxis during interscalene block. This event is so rare as to defy accurate incidence reporting. Other unintended destinations of local anesthetic are better defined. Involvement of the recurrent laryngeal nerve occurs in 1.3% of supraclavicular and 10% of cervical paravertebral blocks using traditional PNS techniques. Anesthesia of the cervicothoracic sympathetic trunk, which manifests as Horner’s syndrome, occurs in 20% to 90% of supraclavicular and 40% of cervical paravertebral blocks using traditional high-volume, PNS-directed techniques.2
Pathophysiology
Neuraxial anesthesia after placement of an interscalene block is caused by either overly deep placement of a medially directed block needle (toward the spinal cord or its meningeal coverings) or by local anesthetic deposition into a long dural root sleeve (Fig. 18-1). Medially directed PNS- or paresthesia-guided needles are described using the Winnie46 technique. Conversely, the modified lateral technique of Borgeat47 relies on a presumably safer superficial needle approach (Chapter 18). Ultrasound-guided interscalene blocks also use a superficial lateral-to-medial needle approach that should theoretically avoid proximity with the neuraxis and all but the longest dural root sleeves.
Hoarseness is directly related to the proximity of the recurrent laryngeal and vagus nerves to the neural components of the brachial plexus. Similarly, the proximity of the cervicothoracic sympathetic trunk to the C8 and T1 nerve roots explains the occurrence of Horner’s syndrome. Although it is reasonable to postulate that these unintended effects of local anesthetic may in part relate to the injected volume, there is no evidence that the 20 to 40 mL volumes used with conventional PNS approaches differentially affect the incidence of recurrent laryngeal or cervicothoracic trunk anesthesia.2 Theoretically, the smaller volumes used with UGRA techniques may reduce the incidence of these nuisance side effects. However, this may not be the case if the effect is related less to the volume and more to the spread along tissue planes.
The Effect of Nerve Localization Technique
With regard to local anesthetic spread to the recurrent laryngeal nerve or the cervicothoracic sympathetic trunk, there are no direct comparisons of these side effects as a function of nerve localization technique. Ultrasound studies that report these side effects suggest that the incidence may be lower than that observed with PNS techniques, most likely secondary to smaller injected volumes of local anesthetic.
 PREVENTION
PREVENTION
Of the previously discussed regional anesthesia complications, it appears that only the incidence of HDP can be reduced considerably by the use of UGRA compared with PNS. Even then, ultrasound does not completely prevent the complication. Therefore, there is no compelling evidence that one’s choice of nerve localization technique can reliably prevent peripheral nerve injury, LAST, HDP, pneumothorax, or complications arising from unintended spread of local anesthetic.
One aspect of prevention is avoiding overconfidence in a technique’s ability to confer an advantage that has yet to be proven. In this paragraph, the author offers his opinion regarding a proposed advantage of UGRA—specifically, intentional intraneural injection (presumably, subepineurial but extraperineurial) for the purpose of improving qualities of neural blockade such as onset and duration. Unintentional intraneural injection at one institution occurred in 17% (95% CI 12%–22%) of patients undergoing ultrasound-guided interscalene and supraclavicular block.48 Clinical observations in a small number of patients have documented intraneural injection without evidence of subsequent injury,13,14,17,48,49 which has led some respected investigators to suggest that intentional intraneural injection may be advantageous.50,51 I and others24,52,53 have challenged this suggestion based on two main arguments: (i) the incidence of peripheral nerve injury is so small as to prohibit conclusions based on a very small number of published reports of apparently harmless intraneural injections and (ii) the spatial resolution of contemporary ultrasound machines is insufficient to distinguish intra- versus extrafascicular needle placement.54 It is thus my opinion that the unknown and probably limited benefits of intentional subepineurial injection are likely less than its unknown but potentially serious risks.52
 SUMMARY
SUMMARY
There is no compelling evidence that nerve localization technique, particularly UGRA or PNS, significantly affects the incidence of major complications such as PONS, LAST, pneumothorax, or unintended destinations of local anesthetic. The two localization techniques are different with regard to the incidence and severity of HDP, with ultrasound guidance providing superior results. However, even this advantage is limited by the absence of total effectiveness in all patients and the unpredictable nature of its effect (Table 17-1).
References
- Neal JM, Brull R, Chan VWS, et al. The ASRA evidence-based medicine assessment of ultrasound-guided regional anesthesia and pain medicine: executive summary. Reg Anesth Pain Med 2010;35:S1–S9.
- Neal JM, Gerancher JC, Hebl JR, et al. Upper extremity regional anesthesia. Essentials of our current understanding, 2008. Reg Anesth Pain Med 2009;34:134–170.
- Perlas A. Evidence for the use of ultrasound in neuraxial blocks. Reg Anesth Pain Med 2010;35:S43–S46.
- Abrahams M, Horn J-L, Noles LM, et al. Evidence-based medicine: ultrasound guidance for truncal blocks. Reg Anesth Pain Med 2010;35:S36–S42.
- Narouze SN. Ultrasound-guided interventional procedures in pain medicine: evidence-based medicine. Reg Anesth Pain Med 2010;35:S55–S58.
- Neal JM, Bernards CM, Hadzic A, et al. ASRA Practice Advisory on neurologic complications in regional anesthesia and pain medicine. Reg Anesth Pain Med 2008;33:404–422.
- Auroy Y, Benhamou D, Bargues L, et al. Major complications of regional anesthesia in France. The SOS regional anesthesia hotline service. Anesthesiology 2002;97:1274–1280.
- Auroy Y, Narchi P, Messiah A, et al. Serious complications related to regional anesthesia. Results of a prospective survey in France. Anesthesiology1997;87:479–486.
- Brull R, McCartney CJL, Chan VWS, et al. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesth Analg 2007;104:965–974.
- Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med 2008;33:435–441.
- Sala-Blanch X, Ribalta T, Rivas E, et al. Structural injury to the human sciatic nerve after intraneural needle insertion. Reg Anesth Pain Med 2009;34:201–205.
- Perlas A, Niazi A, McCartney C, et al. The sensitivity of motor reponses to nerve stimulation and paresthesia for nerve localization as evaluated by ultrasound. Reg Anesth Pain Med 2006;31:445–450.
- Robards C, Hadzic A, Somasundaram L, et al. Intraneural injection with low-current stimulation during popliteal sciatic nerve block. Anesth Analg 2009;109:673–677.
- Bigeleisen PE, Moayeri N, Groen GJ. Extraneural versus intraneural stimulation thresholds during ultrasound-guided supraclavicular block. Anesthesiology 2009;110:1235–1243.
- Chan VW, Brull R, McCartney CJ, et al. An ultrasonic and histologic study of intraneural injection and electrical stimulation in pigs. Anesth Analg 2007;104:1281–1284.
- Altermatt FR, Cummings TJ, Auten KM, et al. Ultrasonographic appearance of intraneural injections in the porcine model. Reg Anesth Pain Med 2010;35:203–206.
- Bigeleisen PE. Nerve puncture and apparent intraneural injection during ultrasound-guided axillary block does not invariably result in neurologic injury. Anesthesiology 2006;105:779–783.
- Lupu CM, Kiehl T-R, Chan VWS, et al. Nerve expansion seen on ultrasound predicts histological but not functional nerve injury following intraneural injection in pigs. Reg Anesth Pain Med 2010;35:132–139.
- Liguori GA, Zayas VM, YaDeau JT, et al. Nerve localization techniques for interscalene brachial plexus blockade: a prospective, randomized comparison of mechanical paresthesia versus electrical stimulation. Anesth Analg 2006;103:761–777.
- Liu SS, Zayas VM, Gordon MA, et al. A prospective, randomized, controlled trial comparing ultrasound versus nerve stimulator guidance for interscalene block for ambulatory shoulder surgery for posoperative neurological symptoms. Anesth Analg 2009;109:265–271.
- Barrington MJ, Watts SA, Gledhill SR, et al. Preliminary results of the Australasian Regional Anaesthesia Collaboration. A prospective audit of over 7000 peripheral nerve and plexus blocks for neurological and other complications. Reg Anesth Pain Med 2009;34:534–541.
- Benhamou D, Auroy Y, Amalberti R. Safety during regional anesthesia: what do we know and how can we improve our practice? (editorial). Reg Anesth Pain Med 2010;35:1–3.
- Orebaugh SL, Williams BA, Vallejo M, et al. Adverse outcomes associated with stimulator-based peripheral nerve blocks with versus without ultrasound visualization. Reg Anesth Pain Med 2009;34:251–255.
- Cohen JM, Gray AT. Functional deficits after intraneural injection during interscalene block. Reg Anesth Pain Med 2010;35:397–399.
- Koff MD, Cohen JA, McIntyre JJ, et al. Severe brachial plexopathy after an ultrasound-guided single-injection nerve block for total shoulder arthroplasty in a patient with multiple sclerosis. Anesthesiology 2008;108:325–328.
- Reiss W, Kurapati S, Shariat A, et al. Nerve injury complicating ultrasound/electrostimulation-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2010;35:400–401.
- Neal JM. Ultrasound-guided regional anesthesia and patient safety: an evidence-based analysis. Reg Anesth Pain Med 2010;35:S59–S67.
- Mulroy MF, Hejtmanek MR. Prevention of local anesthetic systemic toxicity. Reg Anesth Pain Med 2010;35:177–180.
- Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med 2010;35:152–161.
- Abrahams MS, Aziz MF, Fu RF, et al. Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth 2009;102:408–417.
- Casati A, Baciarello M, Di Cianni S, et al. Effects of ultrasound guidance on the minimum effective anaesthetic volume required to block the femoral nerve. Br J Anaesth 2007;98:823–827.
- Di Gregorio G, Neal JM, Rosenquist RW, et al. Clinical presentation of local anesthetic systemic toxicity: a review of published cases, 1979–2009. Reg Anesth Pain Med 2010;35:181–187.
- Tsui BC, Pillay JJ. Evidence-based medicine: assessment of ultrasound imaging for regional anesthesia in infants, children and adolescents. Reg Anesth Pain Med 2010;35:S47–S54.
- Weintraud M, Lundblad M, Kettner S, et al. Ultrasound versus landmark-based technique for ilioinguinal-iliohypogastric nerve blockade in children: the implications on plasma levels of ropivacaine. Anesth Analg 2009;108:1488–1492.
- Urmey WF, Talts KH, Sharrock NE. One hundred percent incidence of hemidiaphragmatic paresis associated with interscalene brachial plexus anesthesia as diagnosed by ultrasonography. Anesth Analg1991;72:498–503.
- Neal JM, Moore JM, Kopacz DJ, et al. Quantitative analysis of respiratory, motor, and sensory function after supraclavicular block. Anesth Analg1998;86:1239–1244.
- Renes SH, Rettig HC, Gielen MJ, et al. Ultrasound-guided low-dose interscalene brachial plexus block reduces the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med 2009;34:498–502.
- Riazi S, Carmichael N, Awad I, et al. Effect of local anaesthetic volume (20 vs 5 ml) on the efficacy and respiratory consequences of ultrasound-guided interscalene brachial plexus block. Br J Anaesth 2008;101:549–556.
- Renes SH, Spoormans HH, Gielen MJ, et al. Hemidiaphragmatic paresis can be avoided in ultrasound-guided supraclavicular brachial plexus block. Reg Anesth Pain Med 2009;34:595–599.
- Urmey W, McDonald M. Hemidiaphragmatic paresis during interscalene brachial plexus block: effects on pulmonary function and chest wall mechanics. Anesth Analg1992;74:352–357.
- Kessler J, Schafhalter-Zoppoth I, Gray AT. An ultrasound study of the phrenic nerve in the posterior cervical triangle: implications for the interscalene brachial plexus block. Reg Anesth Pain Med 2008;33:545–550.
- Sinha SK, Abrams JH, Barnett JT, et al. Decreasing the local anesthetic volume from 20 to 10 mL for ultrasound-guided interscalene block at the cricoid level does not reduce the incidence of hemidiaphragmatic paresis. Reg Anesth Pain Med 2011;36:17–20.
- Perlas A, Lobo G, Lo N, et al. Ultrasound-guided supraclavicular block. Outcome of 510 consecutive cases. Reg Anesth Pain Med 2009;34:171–176.
- Bhatia A, Lai J, Chan VWS, et al. Pneumothorax as a complication of the ultrasound-guided supraclavicular approach for brachial plexus block. Anesth Analg 2010;111:817–819.
- Koscielniak-Nielsen Z, Rasmussen H, Hesselbjerg L. Pneumothorax after an ultrasound-guided lateral sagittal infraclavicular block. Acta Anaesthesiol Scand 2008;52:1176.
- Winnie AP. Interscalene brachial plexus block. Anesth Analg1970;49:455–466.
- Borgeat A, Dullenkopf A, Ekatodramis G, et al. Evaluation of the lateral modified approach for continuous interscalene block after shoulder surgery. Anesthesiology 2003;99:436–442.
- Liu SS, YaDeau JT, Shaw PM, et al. Incidence of unintentional intraneural injection and postoperative neurological complications with ultrasound-guided interscalene and supraclavicular nerve blocks. Anaesthesia2011;66:168–174.
- Sala-Blanch X, Lopez AM, Carazo J, et al. Intraneural injection during nerve stimulator-guided sciatic nerve block at the popliteal fossa. Br J Anaesth 2009;102:855–861.
- Bigeleisen PE, Chelly J. An unsubstantiated condemnation of intra-neural injection. Reg Anesth Pain Med 2011;36:95.
- Rosenblatt MA, Bigeleisen PE. Ultrasound-guided intraneural injection: a powerful tool for regional anesthesia. In: Bigeleisen PE, ed. Ultrasound-Guided Regional Anesthesia and Pain Medicine. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, 2010.
- Neal JM, Wedel DJ.Ultrasound guidance and peripheral nerve injury. Is our vision as sharp as we think it is?Reg Anesth Pain Med 2010;35:335–337.
- Reiss W, Kurapati S, Shariat A, et al. Reply to Drs. Bigeleisen and Chelly. Reg Anesth Pain Med 2011;36:88–99.
- Silvestri E, Martinoli C, Derchi LE, et al. Echotexture of peripheral nerves: correlation between US and histologic findings and criteria to differentiate tendons. Radiology 1995;197:291–296.
< div class='tao-gold-member'>



