Nerve injury
28
Infection
24
Death/brain damage
9
Headache
20
Increased pain, no relief
10
From Fitzgibbon DR, Posner KL,Caplan RA, et al. Chronic pain management: American Society of Anesthesiologists Closed Claims Project. Anesthesiology 2004;100:98–105.
 NEUROTOXICITY
NEUROTOXICITY
The intrathecal injection of neurotoxic substances can result in arachnoiditis or cauda equina syndrome. The dura and arachnoid provide considerable protection against chemical injury to intraspinal neural structures. However, arachnoiditis and neurologic injury can occur if there is sufficient chemical irritation or inflammation. The first reported cases of arachnoiditis were mainly postinfectious and often followed syphilis or tuberculosis infections. Iatrogenic cases became a concern when it was discovered that arachnoiditis could be induced by intrathecal injection of oily radiographic contrast materials. It is most commonly found among patients who have undergone multiple diagnostic and surgical procedures of the spine, and thus it is often difficult to determine the inciting cause. Spine surgery is a recognized etiology.
Arachnoiditis and Cauda Equina Syndrome
Definition
Arachnoiditis is an inflammatory condition involving the leptomeninges and underlying neural structures. The mildest form consists of arachnoid adhesions. Adhesive arachnoiditis is a severe and often progressive form that is associated with neuropathic pain and neurologic dysfunction. Some cases are associated with calcific deposits involving the inflamed meninges and are known as calcific arachnoiditis. The inflammatory response triggers fibrin exudates that cause nerve roots to adhere to each other and to the dural sac. The subsequent repair process produces dense collagen adhesions, hyalinization of the arachnoid membrane, and loss of cerebrospinal fluid (CSF).7 Commonly encountered symptoms are listed in Box 27-2. Radiographic findings are characterized by three patterns, described in Box 27-3.8
BOX 27-2 Symptoms of Adhesive Arachnoiditis
 Constant, burning pain in lower back and legs
Constant, burning pain in lower back and legs
 Urinary frequency, incontinence
Urinary frequency, incontinence
 Muscle spasm, back, and legs
Muscle spasm, back, and legs
 Variable sensory loss
Variable sensory loss
 Variable motor dysfunction
Variable motor dysfunction
BOX 27-3 Radiographic Findings in Arachnoiditis
The following findings can be seen with standard myelography, CT myelography, or with MRI:
 Group 1: Nerve roots adherent centrally in thecal sac.
Group 1: Nerve roots adherent centrally in thecal sac.
 Group 2: Nerve roots tethered peripherally to the dura and arachnoid (empty sac).
Group 2: Nerve roots tethered peripherally to the dura and arachnoid (empty sac).
 Group 3: Inflammatory mass replacing the subarachnoid space.
Group 3: Inflammatory mass replacing the subarachnoid space.
Cauda equina syndrome is a complex and variable entity associated with compression or injury to the lumbar spinal nerve roots that comprise the cauda equina within the thecal sac. The syndrome is characterized by bilateral sciatica, saddle hypesthesia, lower extremity weakness, and bowel, bladder, and sexual dysfunction. Symptoms are confined to the lumbosacral nerve root distribution, but are otherwise indistinguishable from those of arachnoiditis. Cauda equina syndrome can be caused by adhesive arachnoiditis as well as other abnormalities affecting the cauda equina (e.g., external compression of the cauda equina by a large central intervertebral disc herniation, epidural hematoma, or abscess; Fig. 27-1).
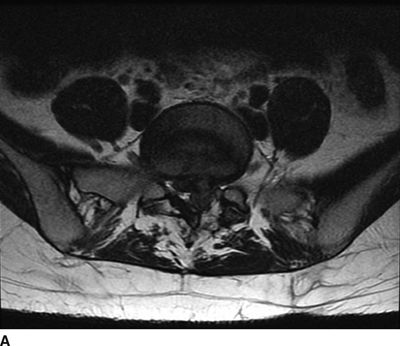
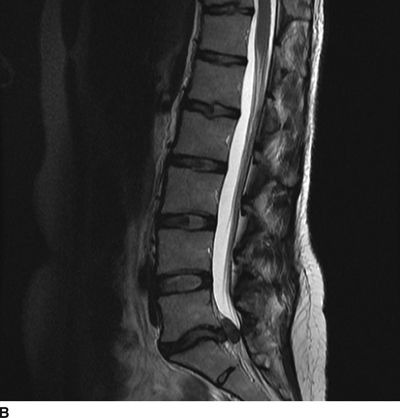
FIGURE 27-1. MRI of the lumbosacral spine demonstrating compression of the cauda equina caused by a large lumbar intervertebral disc herniation. A 31-year-old with a history of prior L5/S1 discectomy underwent an uncomplicated interlaminar epidural steroid injection for treatment of acute radicular pain in the left S1 distribution associated with a recurrent disc herniation. The day following the epidural steroid injection, she presented emergently with new onset of perineal numbness and inability to urinate, raising concern of epidural hematoma related to the injection. Repeat MRI of the lumbosacral spine revealed a large progression in the extent of her central and left-sided L5/S1 disc herniation causing compression of the cauda equina. A: Axial T2-weighted image at the level of L5/S1. B: Sagittal T2-weighted image near midline. (Images provided by J.P. Rathmell.)
Scope
Arachnoiditis and cauda equina syndrome are uncommon conditions and represent a distinct minority of cases among patients with severe back and leg pain. The true prevalence of these conditions among patients who have undergone multiple diagnostic and surgical procedures of the spine is not known.
Pathophysiology
Concern regarding the neurotoxic potential of epidural steroid injections relates almost entirely to the consequences of unintentional intrathecal placement of corticosteroid suspensions. Much of this concern arose from the practice of treating advanced cases of multiple sclerosis (MS) with intrathecal methylprednisolone acetate (MPA). The earliest report of arachnoiditis following intrathecal steroid injections9 cited two cases of adhesive arachnoiditis among a series of 23 patients who received a total of 83 injections of MPA. The author of that report expressed concern that the drug vehicle, polyethylene glycol, initiated the inflammatory response. A third case of arachnoiditis, described by the author as “sclerosing pachymeningitis,” was diagnosed in a patient with advanced MS.10 Prior to steroid therapy, her symptoms included urinary dysfunction, sensory changes in the legs, and upper motor neuron signs. She received six intrathecal injections of MPA over the course of a year, with transient symptomatic improvement and no apparent complications. Her disease progressed dramatically over the following 2 years. She received an additional two injections shortly before her death with no apparent benefit. Postmortem examination revealed severe arachnoiditis with necrotizing and hemorrhagic changes. A fourth case of arachnoiditis that occurred in a patient who received intrathecal MPA treatment for MS occurred after multiple injections over a 2-year period.11 Myelographic findings were compatible with the diagnosis, and thickening and calcification of the meninges were noted when a laminectomy was performed.
One case of myelographically documented arachnoiditis has been reported following intrathecal MPA treatment for lumbar disc disease.12 The patient had a “traumatic tap” during the injection. The arachnoiditis would probably be characterized as group 1 according to the Delamarter classification,8 in that the patient’s symptoms resolved following discectomy. A case of cauda equina syndrome was reported in a patient who received 14 intrathecal injections and 4 epidural injections of MPA over an 18-month period.13 Her initial response to the injections was beneficial and uncomplicated except for the occurrence of transient urinary incontinence following one of the early procedures. After the last injection, she developed persistent sacral anesthesia and urinary retention.
Aseptic Meningitis
Definition
Aseptic meningitis is a generally benign condition that produces signs of neurologic irritation, including burning pain in the legs, headache, meningismus, and in severe cases seizures. Fever and nausea are often reported. CSF examination reveals pleocytosis, elevated protein, and decreased glucose. The introduction of virtually any substance, including normal saline or water, into the subarachnoid space can produce the syndrome.14 Seghal et al.15 documented the occurrence of aseptic meningitis following intrathecal injections of 40 to 200 mg MPA. They found a dose-related polymorphonuclear pleocytosis beginning within 24 hours and lasting about 6 days. At doses over 80 mg, they documented elevation of CSF proteins and clinical signs of meningeal irritation. On the other hand, Goldstein et al.16 were unable to show any CSF changes or symptoms of meningeal irritation in patients with MS treated with 40 mg MPA.
Scope
Several cases of aseptic meningitis have been reported following intrathecal corticosteroid injections.1,17–19 One of these cases was severe, producing headache, fever, nausea, bilateral leg pain, and seizures.1 CSF culture was negative. There was elevation of CSF protein, leukocytes, and red blood cells. Symptoms were prolonged, resolving after 3 weeks. One case of aseptic meningitis was reported following an epidural injection of MPA.20 No local anesthetic was injected with the steroid, and, thus, a dural puncture with intrathecal migration of the drug cannot be ruled out.
Mechanism of Neurotoxicity
It is difficult to determine which component of the steroid preparation, if any, is neurotoxic. Nelson et al.9 suggested that polyethylene glycol is the offending agent. This speculation was based on studies demonstrating that 78% to 80% propylene glycol, an ingredient in a long-acting local anesthetic preparation of the drug efocaine, causes nerve injury.21,22 It has a molecular weight of 78. The polyethylene glycol preparation used in steroid suspensions has a molecular weight of 3,350 and is present in concentrations of 2.8% to 3%. Benzon et al.23 studied the acute effects of polyethylene glycol on nerve conduction in both sheathed and unsheathed neurons in rabbits. They found no functional change with concentrations of 3% and 10% and slowing of conduction with 20% and 30%. A 40% concentration abolished conduction, but the effect was reversible following washout in both sheathed and unsheathed preparations. Benzyl alcohol 0.9% is present in several steroid suspension preparations, including multidose vials of Depo-Medrol brand MPA (Pharmacia & Upjohn, Kalamazoo, MI) and Aristocort Intralesional brand triamcinolone diacetate (American Cyanamid, Madison, NJ). Two animal studies reported on histologic changes following neuraxial injection of the triamcinolone/benzyl alcohol preparation. Delaney et al.24 performed light and electron microscopy studies of nerve roots, cord root entry zone, and meninges after epidural injections of triamcinolone diacetate plus lidocaine in cats. They found mild inflammatory changes 30 days after injection with complete resolution by 120 days. Abram25 examined the spinal cord and meninges of rats injected intrathecally four times at 5-day intervals with triamcinolone diacetate. Animals demonstrated no neurologic dysfunction. There were no histologic differences between steroid and saline-injected animals. There have been reports of aseptic meningitis following intracisternal injections of pyrogen-free serum albumen plus 0.9% benzyl alcohol.26 Deland27 therefore assessed the effects of intracisternal injections of benzyl alcohol 0.9% to 9% in dogs. The highest concentration (10 times the concentration used as a preservative in pharmaceutical agents) produced transient neurologic dysfunction related to local anesthetic effects, but there was no evidence of aseptic meningitis at any concentration. Few histologic abnormalities were noted, and these were seen as frequently in saline controls. Hahn et al.28 reported a case of motor blockade following intrathecal cancer chemotherapy with cytosine arabinoside diluted in bacteriostatic water containing 1.5% benzyl alcohol. Function returned to normal after washout with saline. The authors subsequently showed that benzyl alcohol caused nerve root injury in animal studies.
In Australia, where there was substantial public controversy about the risk of arachnoiditis following epidural MPA injection during the 1990s, some physicians have begun to use Celestone Chronodose (Schering-Plough, Kenilworth, NJ) for steroid epidurals. This product contains betamethasone 5.7 mg, betamethasone sodium phosphate 3.9 mg (in solution), and betamethasone acetate 3 mg (in suspension) per milliliter in an aqueous vehicle-containing sodium phosphate, sodium phosphate monobasic, disodium edetate, benzalkonium chloride, and water. Despite the absence of both polyethylene glycol and benzyl alcohol, a study in sheep demonstrated the development of histopathologic changes of arachnoiditis following intrathecal injection of 2 mL or more of this preparation.29 The product is available in the United States as Celestone Soluspan (Schering-Plough, Kenilworth, NJ). As is the case with all of the commercially available corticosteroid suspensions, it does not have a product indication for epidural injection for the treatment of sciatica.
Prevention of Neurotoxicity
It is not clear whether a single intrathecal injection is likely to cause serious harm. As noted previously, the reported cases of arachnoiditis were associated with multiple intrathecal injections, and in most cases there was preexisting neurologic disease. A recent study of intrathecal MPA for postherpetic neuralgia failed to find any evidence of either aseptic meningitis or arachnoiditis among 89 patients treated with four 60-mg injections.30 Patients were followed for 2 years and underwent diagnostic lumbar punctures and MRIs. On the other hand, arachnoiditis, cauda equina syndrome, and aseptic meningitis are complications of intrathecal, not epidural, steroid injections. The surest way to prevent their occurrence is to see that the injected drugs are not administered intrathecally. The use of a test dose of local anesthetic will help prevent intrathecal administration. Likewise, fluoroscopic guidance and injection of contrast can be helpful, as the pattern of intrathecal dye spread can be distinguished from that of epidural spread. If accidental dural puncture occurs, epidural injection at another level can still result in intrathecal spread of the injected drug. There is yet another reason to temporarily abandon the procedure if accidental dural puncture occurs. It is likely that flow of CSF into the epidural space will dilute the injected steroid or will carry it away from the targeted neural structures.
Treatment of Neurotoxicity
There is no definitive treatment for arachnoiditis. Symptomatic treatment is the same as for other forms of neuropathic pain. For aseptic meningitis, symptomatic treatment and reassurance are all that is required.
 NEUROLOGIC INJURY
NEUROLOGIC INJURY
Scope and Mechanism of Injury
In the Closed Claims Study, nerve injury occurred in 14 patients following epidural steroid injection.6 Six of these resulted in paraplegia and one in quadriplegia. Spinal cord damage can occur from needle entry into the cord. Such injuries are generally mild unless there is bleeding into the cord. More severe injury will occur if material is injected into the substance of the spinal cord. Another mechanism of injury is injection of steroid suspension into a radicular artery with embolization of end arteries in the spinal cord. This type of injury was implicated in a fatal case of massive cerebellar injury following a transforaminal injection of triamcinolone acetonide.31 The authors of this case report demonstrated that all of the corticosteroid suspensions they tested contained particles large enough to occlude capillaries and arterioles. Embolization has not been implicated as a mechanism for injury following caudal or interlaminar epidural steroid injections. However, there are arteries in the posterior epidural and subarachnoid spaces that communicate with those supplying the cord, and embolization with suspended corticosteroid material is theoretically possible. While intravascular dye spread has been documented during interlaminar cervical epidural steroid injection,32 all of the reported cases were documented as venous injections, which would not be expected to produce any neurologic injury.
Embolization of a radicular artery is theoretically possible during cervical facet injection with particulate steroid preparations. Heckman et al.33 reported a case of transient quadriplegia following a C5-6 facet injection using 1% lidocaine. They postulated radicular artery injection as the cause. The procedure was done without fluoroscopy, so the final needle position could not be determined. Undoubtedly, the outcome would have been disastrous had particulate steroid been included in the injectate.
Direct Spinal Cord Trauma
Needle injury or injection into the cord is a significant risk for cervical, thoracic, and upper lumbar epidural injections. Two cases of spinal cord injury following cervical epidural steroid injections were reported by Hodges et al.34 Both cases used fluoroscopic guidance, both cases were performed at C5-6, and in both cases the patient was sedated with a combination of midazolam and propofol. It was postulated that the patients failed to respond to needle contact with the cord because of the sedation. Both patients experienced persistent upper extremity pain and lower extremity paresthesias. Four unreported cases of spinal cord injury resulted in litigation in which the author served as an expert witness (all of these claims are now closed). The first resulted from a cervical epidural steroid injection performed in an anesthetized patient that resulted in a fatal injury to the upper cervical cord and medulla. Two additional cervical epidural steroid injections resulted in significant and permanent pain and neurologic injury. All of these cervical procedures were performed at C5-6 or above, and all involved the use of moderate to deep sedation. The fourth case was a lumbar epidural steroid injection performed without fluoroscopic guidance. The patient received general anesthesia because of allergy to local anesthetic. Complete and permanent motor and sensory block of one leg became evident immediately after the procedure. MRI showed a new lesion in the conus. Field et al.35 reported three cases of transient neurologic injury that followed otherwise uneventful cervical epidural steroid injections in awake patients. All three patients had large disc herniations that caused effacement of the epidural fat and spinal fluid surrounding the spinal cord at the level of injection (Fig. 27-2). The authors hypothesized that direct injury to the spinal cord or dorsal nerve root could occur even without dural puncture when narrowing or obliteration of the epidural space caused by a large disc herniation displaces the spinal cord posteriorly.
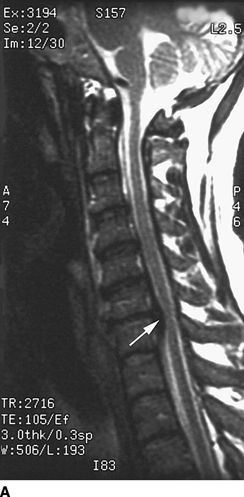
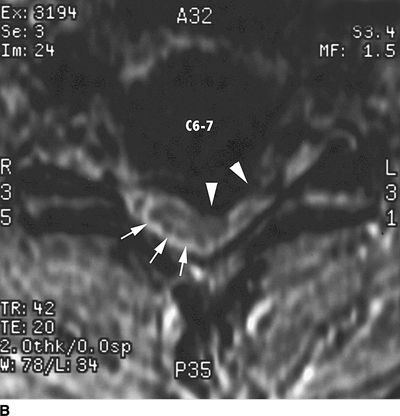
FIGURE 27-2. Field et al.35 reported three cases of transient neurologic injury that followed otherwise uneventful cervical epidural steroid injections in awake patients. All three patients had large disc herniations causing effacement of the epidural fat and spinal fluid surrounding the spinal cord at the level of injection. A: Midline sagittal T2-weighted MRI showing large disc herniation at the C6/7 level (arrow) that effaces the epidural fat and CSF signal both anterior and posterior to the spinal cord. B: Axial T1-weighted MRI showing a large central and left-sided disc herniation (arrowheads) displacing the spinal cord (arrows) to the right posterolateral limits of the spinal canal. Reproduced from Field J, Rathmell JP, Stephenson JH, et al. Neuropathic pain following cervical epidural steroid injection. Anesthesiology 2000;93:885–888, with permission.
Prevention of Direct Spinal Cord Trauma
There is enough evidence from case reports of direct trauma to the spinal cord following epidural steroid injections to suggest the following guidelines:
1. Examine MRI images before the procedure. Avoid injection at cervical or thoracic levels where the spinal cord has been displaced posteriorly. Consider advancing a catheter from below in such situations.
2. Avoid entry into the epidural space above C6-7. MRI scans and cryomicrotome anatomic studies indicate that there is no space between the ligamentum flavum and the dura at C5-6 and above.
3. Obtain a lateral fluoroscopic view before injecting anything. Ensure that the needle is in the most dorsal aspect of the epidural space. In the low-cervical spine, the shoulders may obscure the lateral view. The use of the “swimmer’s view” (one arm raised 180 degrees overhead and the other arm at the side) may allow visualization of the needle and the spine.36
4. Avoid deep sedation or general anesthesia during the procedure. The patient should be alert enough to respond to paresthesias induced by needle contact with neural structures, though this precaution does not guarantee safety.Simon et al.37 reported a case of intramedullary injection of contrast during a C1-2 myelogram. A total of 15 mL iohexol was injected, resulting in unilateral arm weakness and diffuse hyperreflexia. Presumably, the patient was awake (there was no mention of sedation) and reported no sensation during needle placement but experienced paresthesias in the face, neck, and arm during injection of the dye. Similarly, Tripathi et al.38 reported a case of cord injury following a T11-12 epidural steroid injection in a nonsedated patient who experienced no paresthesias during needle placement or during injection.
Treatment of Direct Spinal Cord Trauma
In most cases, there is probably little that can be done to minimize the extent of neurologic dysfunction after the traumatic event has occurred. High-dose intravenous corticosteroid may be of benefit. High-dose intravenous steroids administered in the hours immediately following traumatic spinal cord injury have been shown to result in a significant reduction in neuronal injury.39 The patient who experienced intramedullary iohexol was treated promptly with an intravenous bolus of 30 mg/kg methylprednisolone followed by 5.4 mg/kg/h for 48 hours. Her symptoms reportedly began to improve within 4 hours of steroid treatment.39
 PHARMACOLOGIC EFFECT OF CORTICOSTEROIDS
PHARMACOLOGIC EFFECT OF CORTICOSTEROIDS
Hypercorticism and Adrenal Suppression
Scope and Pathophysiology
Cushing syndrome, a characteristic pattern of obesity with associated hypertension, is the result of abnormally high blood levels of cortisol resulting from hyperfunction of the adrenal cortex. Prolonged exogenous administration of glucocorticoids results in a clinical pattern identical to the spontaneous disorder and is frequently called cushingoid syndrome. The active corticosteroid in MPA and other depot steroid preparations is slowly released over a period of days to 1 to 2 weeks. It is common for patients to report side effects, mostly during the first 3 posttreatment days. Fluid retention and weight gain40 as well as increased blood pressure36 and congestive heart failure41 have been reported after epidural steroid injections. Cushingoid side effects, usually beginning 1 to several weeks after epidural steroid injections, have been reported by several authors.42–44 These include facial swelling, buffalo hump, skin bruising, and scaly skin lesions. The most dramatic case occurred in a patient who had received a single cervical epidural injection of 60 mg MPA. Over the following month, he developed a 20-lb weight gain, moon facies, buffalo hump, and neck thickening. These symptoms persisted for 1 year. Laboratory investigation showed markedly reduced serum cortisol levels and no response to adrenocorticotropic hormone (ACTH). Cortisol levels rose slowly, but were still low (normal at 6 months). Jacobs et al.45 documented marked suppression of plasma cortisol levels in 12 patients who each received a single epidural injection of 80 mg MPA. Plasma cortisol and ACTH levels were significantly depressed at 1, 7, 14, and 21 days after treatment. The ability of exogenous ACTH to increase plasma cortisol levels was also reduced over a 3-week period. Kay et al.46 assessed the adrenal response to a series of three weekly epidural injections of 80 mg triamcinolone diacetate. Suppression of serum cortisol and ACTH began within 45 minutes of the initial injection and remained low 7 days after each of the first two injections. Levels were nearly normal 30 days after the last injection. Patients sedated with midazolam during the procedures had greater and more prolonged suppression. Another symptom of hypercorticism is steroid-induced myopathy, which is characterized by progressive proximal muscle weakness, increased serum creatinine kinase levels, and a myopathic electromyogram (EMG) and muscle biopsy specimen. This has been reported following a single epidural dose of triamcinolone.47 All patients who have been taking steroids for long periods develop reversible myofiber atrophy, but this is not steroid myopathy unless it progresses to become a necrotizing myopathy.
Prevention of Hypercorticism and Adrenal Suppression
Because the most severe reported case of Cushing syndrome and adrenal suppression occurred after a single, relatively small steroid dose, it is unlikely that this complication can be avoided in susceptible patients. Repeat procedures should not be done for patients who have persistent improvement following a single injection.
Treatment of Hypercorticism and Adrenal Suppression
Patients undergoing surgery within a few weeks of receiving depot steroids should be evaluated for adrenal suppression or should receive steroid coverage during the perioperative period. Otherwise, no specific treatment is indicated.
Epidural Lipomatosis
Definition and Scope
An increase in the amount of fat in the epidural space can be seen in some patients who have been treated with systemic corticosteroids (see Chapter 10). There is concern that epidural steroid administration can produce similar increases in the amount of epidural fat. Although this is probably a rare phenomenon, there is some evidence that epidural lipomatosis can be caused or aggravated by epidural steroid injection. Sandberg and Lavyne48 published a case report of a 68-year-old man who presented with right sciatica and MRI documentation of spinal stenosis but normal volume of epidural fat. He was treated with two epidural injections, 1 month apart, of MPA 120 mg. Three years later, he presented with bilateral sciatica and neural claudication. MRI demonstrated persistent spinal stenosis and mild epidural lipomatosis. He underwent three injections, a month apart, of triamcinolone diacetate 80 mg. He experienced transient improvement after each injection and then persistent worsening of symptoms. MRI 3 months after the last injection showed a substantial increase in epidural fat from L2 to L5, with compression of the thecal sac. He underwent multilevel laminectomy and removal of epidural fat with subsequent improvement in symptoms. Another case report discussed a patient who had been on chronic steroid treatment for asthma and who was treated with epidural steroids without prior spine imaging. A subsequent MRI demonstrated severe epidural lipomatosis.49 It was not known if the condition was exacerbated by the epidural injection.
Diagnosis, Prevention, and Treatment
Epidural lipomatosis is an unlikely complication of epidural steroid injections. However, it should be ruled out by MRI in patients with a history of chronic steroid use before proceeding.
Allergy to Corticosteroids
Although rare, allergic reaction to steroid suspension has been documented. Simon et al.50 reported a delayed allergic reaction to triamcinolone diacetate that began a week after an epidural steroid injection. Following the patient’s recovery, skin testing resulted in recurrence of symptoms after 12 hours.
Altered Glucose Tolerance
Definition and Scope
Glucocorticoid administration reduces the hypoglycemic effect of insulin51 and interferes with blood glucose control in diabetic patients. Following injection of depot steroids, diabetic patients generally report increased blood glucose levels and insulin requirements for 48 to 72 hours. Surprisingly, there is almost no medical literature on the effect of long-acting steroid administration on blood sugar control in diabetics. There is some information available on the effect of exogenous corticosteroids on glucose metabolism in normal patients.
Pellacani et al.52 studied the effect of two doses of 40 mg soluble methylprednisolone administered intravenously 12 hours apart. They documented a significant reduction in glucose tolerance 2 hours after the second injection with return to normal by 24 hours. Injection of MPA or other steroid suspensions would be expected to have a less profound but more prolonged effect. Ward et al.53 studied the metabolic effect of a single caudal injection of triamcinolone acetonide. They found that fasting glucose levels and serum glucose response to insulin were significantly depressed, and serum insulin levels were significantly increased 24 hours after injection. All three values had returned to normal after 1 week.
Treatment of Altered Glucose Tolerance
Glucose levels in diabetic patients should be monitored closely during the week following any type of depot steroid injection. Patients need to be informed that adjustment of insulin dose may be required. Brittle diabetics should consult their internist or endocrinologist prior to initiating steroid treatment.
Other Side Effects and Complications of Corticosteroid Therapy
Both short and long-term corticosteroid therapies are associated with a substantial number of adverse effects. Although many of these side effects and complications have not been reported following injections of steroid suspensions, most are possible, particularly if injections are repeated over prolonged intervals. Box 27-4 provides a list of many of these adverse effects.
BOX 27-4 Adverse Systemic Effects of Corticosteroids
 Glucose intolerance
Glucose intolerance
 Hypokalemia
Hypokalemia
 Hypertension
Hypertension
 Myopathy
Myopathy
 Cutaneous changes
Cutaneous changes
 Truncal obesity
Truncal obesity
 Pancreatitis
Pancreatitis
 Psychotic reactions
Psychotic reactions
 Dementia
Dementia
 Benign intracranial hypertension
Benign intracranial hypertension
 Seizures
Seizures
 Osteoporosis
Osteoporosis
 Aseptic necrosis, femoral, or humeral head
Aseptic necrosis, femoral, or humeral head
 Adrenal insufficiency
Adrenal insufficiency
 Increased intraocular pressure
Increased intraocular pressure
 Cataract
Cataract
 Hyperlipidemia
Hyperlipidemia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


