DEFINITION
Infectious complications may occur after any regional anesthetic technique but are of greatest concern if the infection occurs near or within the neuraxis. The possible risk factors include underlying sepsis, diabetes, depressed immune status, steroid therapy, localized bacterial colonization or infection, and prolonged catheter duration. Bacterial infection within the neuraxis may present either as meningitis or with cord compression secondary to abscess formation. The infectious source may be exogenous (as from contaminated equipment or medication) or endogenous (seeding from a bacterial source to the needle or catheter site). Microorganisms can also be transmitted via a break in aseptic technique, and indwelling catheters may be colonized from superficial skin pathogens, which subsequently spread to the epidural or intrathecal space. Peripheral nerve blocks, with or without catheter placement, may also rarely be associated with infectious sequelae.
 SCOPE
SCOPE
Although individual cases have been reported, serious neuraxial infections such as meningitis and abscess following spinal or epidural anesthesia are fortunately rare (Table 5-1). Infectious complications following peripheral nerve blockade are even less common. In the early 1980s, a combined series of more than 65,000 spinal anesthetics reported only three cases of meningitis and failed to disclose a single epidural or intrathecal infection in approximately 50,000 epidural anesthetics.1 In 1997, a French multicenter prospective study by Auroy et al. that included 40,640 spinal and 30,413 epidural anesthetics reported no infectious complications.2 Several recent large Scandinavian studies have attempted to define the risk in their populations by reviewing large databases with known denominators. Aromaa et al.3 reported eight cases of bacterial infections in Finnish patients undergoing 170,000 epidural and 550,000 spinal anesthetics (1.1:100,000 blocks). A matter of concern is that Wang et al’s.4 Danish survey estimated the incidence of epidural abscess following epidural catheter to be 1:1,930 cases. Finally, Moen et al.5 reviewed the Swedish experience from 1990 to 1999, reporting a low incidence of epidural abscess but an alarming association of postspinal block meningitis with alpha-hemolytic streptococcal cultures, suggesting a nosocomial origin.
TABLE 5.1 Infectious Complications Following Neuraxial Anesthesia
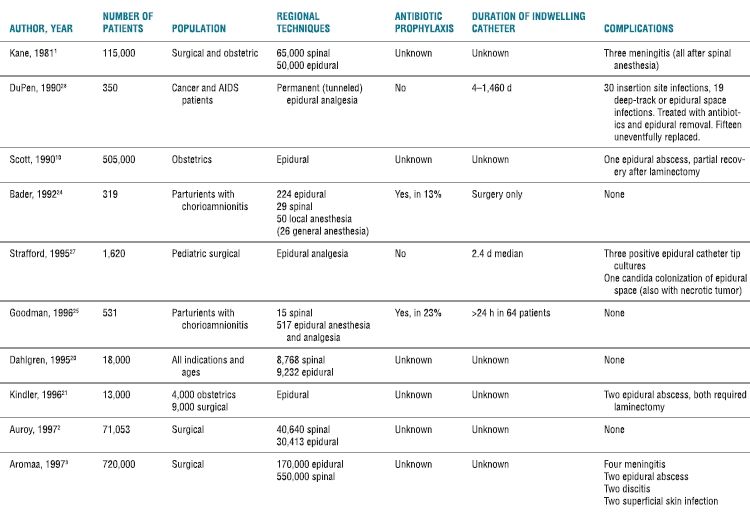
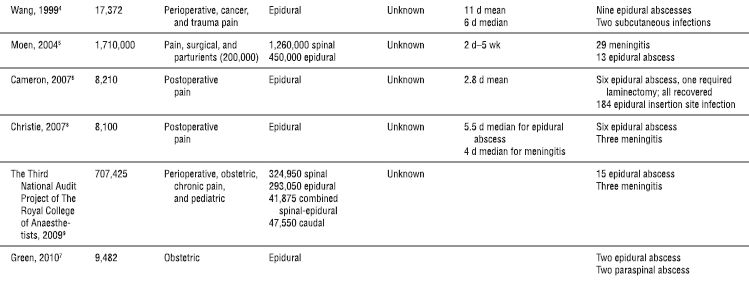
More recent data from multiple reports worldwide confirm the infrequent incidence of major infectious complications following neuraxial blockade, but echo this wide variability in frequency. In Australia, epidural abscesses were identified at a rate of 1:1,368 in patients receiving epidural analgesia for acute postoperative pain,6 and 1:4,742 in women who received an epidural for labor and delivery.7 A United Kingdom (UK) 5-year retrospective review of epidural catheters placed for postoperative analgesia in a cohort of 8,100 patients reported six cases of epidural abscess (1:1,350) and three cases of meningitis (1:2,700).8 A subsequent nationwide audit of major complications after epidural, subarachnoid, caudal, and combined spinal/epidural techniques in the UK was performed. Fifteen cases of epidural abscess and three cases of meningitis were identified in an estimated 707,425 procedures annually (1:39,301).9 Of note, epidural analgesia was found to have a significantly higher risk of infectious complications when compared to spinal anesthesia.
The obstetrical patient group is an interesting subset, with epidural-related infections being extremely rare. Scott and Hibbard10 reported only a single epidural abscess in 505,000 epidurals for obstetrical analgesia and anesthesia over a 4-year period in the UK. Moen et al.5 also noted a significantly lower incidence of infectious complications following epidural anesthesia in the obstetrical population (1:25,000) compared to the nonobstetrical population (1:3,600). A more recent retrospective chart review of 9,482 epidural placements in obstetric patients by Green and Paech7 from a major teaching hospital in Australia reported two epidural abscesses (1:4,741). There was no comparison to nonobstetric epidural catheter placement in this study. Relatively short catheter durations and lack of immunocompromise in this generally healthy population are factors that may contribute to the apparently lower incidence of infectious complications.
 PATHOPHYSIOLOGY
PATHOPHYSIOLOGY
Meningitis
Meningeal puncture has long been considered a risk factor in the pathogenesis of meningitis (Table 5-2). Exactly how microbes cross from the bloodstream into the spinal fluid is unknown. The suggested mechanisms include the introduction of blood into the intrathecal space during needle placement and/or the disruption of the protection provided by the blood-brain barrier. However, lumbar puncture is often performed in patients with fever or infection of unknown origin. If dural puncture during bacteremia results in meningitis, definite clinical data should exist. In fact, clinical studies are sparse and often outdated.
TABLE 5.2 Meningitis After Dural Puncture
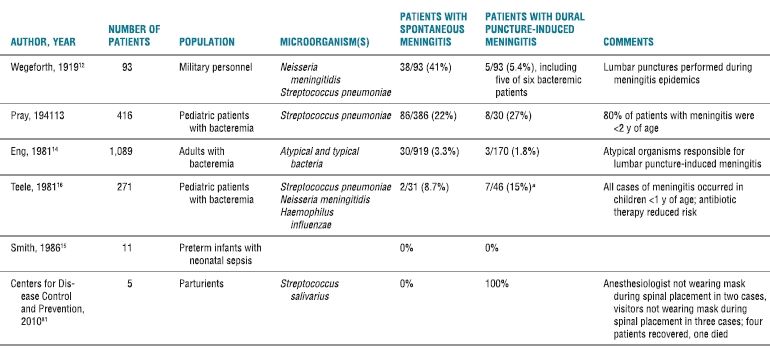
aSignificant association (p < .001).
Spontaneous meningitis = concurrent bacteremia and meningitis (without a preceding lumbar puncture).
Lumbar puncture-induced meningitis = positive blood culture with sterile CSF on initial exam; subsequent positive CSF culture (same organism present in the blood).
Early laboratory and clinical investigations date back to the early 1900s (Table 5-1), when Weed et al.11 demonstrated in an animal model that lumbar or cisternal puncture performed during septicemia (produced by lethal doses of IV-administered gram-negative bacillus) invariably resulted in a fatal meningitis. In the same year, Wegeforth and Latham12 reported their clinical observations on 93 patients with suspected meningitis who received a diagnostic lumbar puncture. Blood cultures were taken simultaneously. The diagnosis of meningitis was confirmed in 38 patients. The remaining 55 patients had normal cerebrospinal fluid (CSF). However, 6 of these 55 patients were bacteremic at the time of lumbar puncture, and 5 of the 6 patients subsequently developed meningitis. It was implied, but not stated, that patients with both sterile blood and CSF cultures at the time of puncture did not develop meningitis. Unfortunately, these lumbar punctures were performed during two epidemics of meningitis at a military installation, and it is possible that some (or all) of these patients may have developed meningitis without lumbar puncture. These two historical studies provided support for the claim that lumbar puncture during bacteremia was a risk factor for meningitis. Subsequent clinical studies reported conflicting results. In two studies of bacteremic patients undergoing diagnostic lumbar puncture, the incidence of meningitis was no greater among patients subjected to lumbar puncture than those who were not13; and there was also no difference in the incidence of postlumbar puncture meningitis compared to spontaneous meningitis.14 The authors concluded: “If lumbar puncture induced meningitis does occur, it is rare enough to be clinically insignificant.”14 In contrast, a review of meningitis associated with serial lumbar punctures to treat posthemorrhagic hydrocephalus in premature infants implicated bacteremia (typically associated with central venous or umbilical artery catheterization) as a risk factor for developing meningitis.15 Results regarding the relative risk of repeated dural punctures, difficult or traumatic procedures, and the use of antibiotics around the time of the lumbar puncture are mixed and likely affected by treatment bias.15,16
Although the prevention of lumbar puncture-induced meningitis with antibiotic therapy seems intuitively possible, there are limited clinically applicable data in the literature. In a more modern version of the animal study performed in the early 1900s, 12 of 40 rats subjected to cisternal puncture during Escherichia coli bacteremia subsequently developed meningitis.17 Meningitis occurred only in animals with blood cultures documenting >50 colony-forming units/mL at the time of dural puncture, a circulating bacterial count observed in patients with infective endocarditis. However, bacteremic rats treated with a single dose of gentamicin immediately prior to cisternal puncture did not develop meningitis. Unfortunately, this study did not include a group of animals that were treated with antibiotics after dural puncture, which would more closely mirror the timing of prophylactic antibiotic administration in a typical operating room. In addition, although E. coli is a common cause of bacteremia, it is an uncommon cause of meningitis in humans. Also, the authors knew the sensitivity of the bacteria injected, allowing for appropriate antibiotic coverage. Although these results may apply to the performance of spinal anesthesia in the bacteremic patient, they do not apply to administration of epidural anesthesia in the febrile patient, which is associated with a higher incidence of infectious complications. While bacteremia may well be a factor in the development of meningitis following lumbar puncture, it is difficult to quantify the specific risk. Multiple factors, including the type of infectious organism, the timing and choice of concurrent antibiotic therapy, and patient immune status all contribute to the individual patient’s unique risk profile.
Epidural Abscess
The incidence of epidural abscess was historically reported to be extremely low. However, several recent studies have shown alarming incidence rates near 1:1,350,4,6–8 while others suggest that the occurrence is rare.5,9 Differences in patient selection, local practices, study design, and statistical analysis may contribute to this variation. Because of this, a true risk estimate for each individual patient may be difficult to ascertain.
Several studies have specifically examined the risk of epidural abscess in patients receiving epidural anesthesia and/or analgesia (Box 5-1). In a historical large retrospective review, epidural abscess from all causes accounted for 0.2 to 1.2 cases per 10,000 admissions to tertiary hospitals.18 Of the 39 cases of epidural abscess occurring over a 30-year period from 1947 to 1974, Staphylococcus aureus (57%), streptococci (18%), and gram-negative bacilli (13%) were the most common pathogens. The source of infection was most often due to osteomyelitis (38%), bacteremia (26%), and postoperative infection (16%). Only 1 of the 39 cases was related to an epidural catheter. In a more recent review, 4 of 10 cases of epidural abscess reported from a Scandanavian database were associated with neuraxial procedures, including repeated lumbar punctures in the presence of meningitis (two cases), epidural catheter placement (one case), and a paravertebral anesthetic injection (one case).19 As with the meningitis data, variability in the incidence of this devastating complication was observed in the recent literature. Retrospective reviews reporting a low incidence of epidural abscess include a Swedish study reporting 0 in 9,232 epidurals20 and a German report of 2 cases in 13,000 procedures.21 However, these reassuring studies notwithstanding, Wang et al.4 present a differing view in the results of their 1-year prospective survey of Danish anesthesiologists. Seventy-eight percent of anesthesia departments participated, performing 17,372 epidural anesthetics. Twelve possible epidural abscesses were reported; nine were subsequently determined to be spinal-epidural abscesses, two were subcutaneous infections, and one was a misplaced catheter. The nine abscesses represented an incidence of 1:1,930 catheters and differed between university (1:5,661) and nonuniversity community hospitals (1:796). The epidural catheters in the affected cases were in situ for a mean of 11 days. Five of the nine involved thoracic catheters, and 67% were placed for perioperative pain management. The majority (67%) had received low–molecular-weight heparin (LMWH) as thromboprophylaxis before epidural catheter placement, and all but one patient were deemed immunocompromised. S. aureus was the pathogen in six of the nine cases (two patients had no bacterial growth). Several common factors, with undetermined significance, were noted in the affected patients: longer mean catheter times, immunocompromised patients with chronic disease states, and perioperative antithrombotic agents were administered in the majority. The authors also pointed out that the overall neurological outcome in these patients was grave, perhaps due to the insidious progression of symptoms and often late intervention (Box 5-2). The diagnosis of epidural abscess is more difficult and often delayed in patients with chronic epidural abscesses because these patients are less likely to be febrile or have elevated leukocyte counts compared to patients with acute abscesses. However, rapid neurologic deterioration may still occur in either group. In addition, earlier diagnosis and treatment improves the neurologic outcome. Steroid administration and increased neurologic impairment at the time of surgery adversely affects the outcome.22
BOX 5-1 Clinical Challenges and Considerations in Immunocompromised Patients
 The range of microorganisms causing invasive infection in the compromised host is much broader than that affecting the general population.
The range of microorganisms causing invasive infection in the compromised host is much broader than that affecting the general population.
 The altered inflammatory response within the compromised patient may mute the clinical signs and symptoms often associated with infection, delaying diagnosis, and treatment.
The altered inflammatory response within the compromised patient may mute the clinical signs and symptoms often associated with infection, delaying diagnosis, and treatment.
 A major factor in determining the prognosis and outcome of immunocompromised patients is the institution of early and effective therapy.
A major factor in determining the prognosis and outcome of immunocompromised patients is the institution of early and effective therapy.
 Prolonged therapy is often required because of persistent and ongoing host defense abnormalities.
Prolonged therapy is often required because of persistent and ongoing host defense abnormalities.
 Prevention of infection should be the goal in managing immunocompromised patients.
Prevention of infection should be the goal in managing immunocompromised patients.
BOX 5-2 Factors That May Increase the Risk for Neuraxial Infection
 Prolonged catheter in situ
Prolonged catheter in situ
 Immunocompromised patient
Immunocompromised patient
 Thromboprophylaxis
Thromboprophylaxis
 Chronically ill patient
Chronically ill patient
 Breaks in aseptic technique
Breaks in aseptic technique
 Preexisting infection of skin, soft tissues, or spine at the insertion site
Preexisting infection of skin, soft tissues, or spine at the insertion site
 Epidural placement (versus spinal)
Epidural placement (versus spinal)
Although infection has long been a concern in epidural anesthesia and analgesia, most cases of epidural catheter-induced spinal epidural abscess or meningitis appear as individual case reports or in retrospective reviews.3,5,18,19,23 Although epidural catheters frequently show positive cultures, clinical signs of epidural infection at the time of catheter removal or during follow-up are rare. Most epidural abscesses are not due to contamination during the placement of indwelling catheters, but appear to be related to preexisting infections of the skin, soft tissue, or spine, or hematogenous spread to the epidural space. Longer catheter duration for postoperative pain management, immunocompromised host, and concurrent anticoagulation seem likely to be contributors to a higher observed incidence in some studies.
Obstetrics
Once again the obstetric patient presents a unique challenge. The anesthesiologist is frequently faced with the management of the parturient with suspected chorioamnionitis, approximately 8% of whom are bacteremic.24,25 These data are derived from two seminal studies in the literature describing the outcome of epidural catheter placement in bacteremic parturients with and without antibiotic treatment. Bader et al.24 investigated the use of regional anesthesia in women with chorioamnionitis. Three hundred nineteen women were identified from a total of 10,047 deliveries. Of the 319 women, 100 had blood cultures taken on the day of delivery. Eight of these had blood cultures consistent with bacteremia. Two hundred ninety three of the 319 patients received a regional anesthetic (in 43 patients, antibiotics were administered prior to needle or catheter placement). Goodman et al.25 also retrospectively reviewed the hospital records of 531 parturients who received epidural or spinal anesthesia and were subsequently diagnosed with chorioamnionitis. Blood cultures were drawn in 146 patients (13 were positive). Antibiotics were administered before the regional block was placed in only 123 patients, whereas nearly one-third of patients did not receive antibiotic therapy in the entire peripartum period. As with the study by Bader et al.24, leukocytosis, fever, abdominal tenderness, or foul-smelling discharge were not predictors of positive blood cultures. No patient in either study, including those with documented bacteremias, had infectious complications. The authors concluded that administering spinal and epidural anesthesia in patients with suspected chorioamnionitis was acceptable because the potential benefits of regional anesthesia outweighed the small theoretical risk of infectious complications. However, the relatively small number of patients with documented bacteremias in both studies precludes a definitive statement regarding the risk of CNS infections in patients suspected of chorioamnionitis undergoing regional anesthetic techniques.
Pediatrics
Studies focusing on pediatric patients report similar findings to those in adults. Specifically, longer catheter duration and placement for treatment of chronic pain (3.2%) rather than acute postoperative pain (0.057%) are risk factors for developing neuraxial infection.26,27 Vigilant monitoring for early signs of infection and early intervention to avoid serious neurologic sequelae are critical to a good outcome.
Technical issues during neuraxial blockade have been implicated as sources of infection. The catheter hub, catheter insertion site, and hematogenous spread are three major routes of entry for microorganisms into the epidural space, with the catheter hub accounting for nearly half of the sources (Fig. 5-1).28–30 A bacterial filter placed at the catheter hub acts as a physical barrier to bacteria present in the infusing solution and should theoretically reduce the incidence of epidural colonization. However, studies of epidural catheter tip cultures have reported mixed results, and cases of epidural infection following hub colonization despite the use of filters have been reported.28,29,31 Possible explanations for hub-related epidural infections in patients with bacterial filters include a reduced antimicrobial effectiveness with prolonged use and direct contamination of the hub during filter-changing techniques. A positive trend between the number of filter changes and the rate of positive hub cultures has been reported.32 These data suggest that continued close attention to aseptic technique is warranted throughout the period of epidural catheterization and that the use of bacteriologic filters alone is unlikely to be efficacious in preventing epidural colonization and infection.33 Overall, epidural abscess following neuraxial anesthetic procedures is uncommon, with the most commonly reported incidence near 1:40,000, and tends to arise from the introduction of nosocomial organisms during the procedure itself or via the contamination of an indwelling catheter.
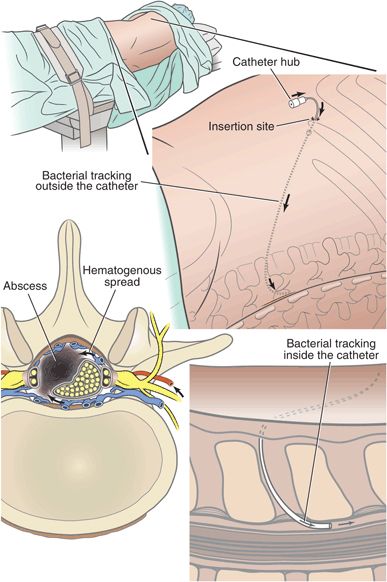
FIGURE 5-1. Bacteria may gain access to the epidural space through the catheter hub, the catheter insertion site, or via hematogenous spread.
 RISK FACTORS
RISK FACTORS
Meningitis after spinal anesthesia has been only rarely reported. In an older study evaluating the frequency of meningitis in patients undergoing spinal anesthesia, Kilpatrick and Girgis34 retrospectively reviewed the records of all patients admitted to the meningitis ward in Cairo, Egypt. During a 5-year period from 1975 to 1980, 17 of 1,429 patients admitted with a diagnosis of meningitis had a history of recent spinal anesthesia. Ten of the 17 had positive CSF cultures: eight were Pseudomonas aeruginosa, one was S. aureus, and one was S. mitis. These organisms were not cultured from patients who had not undergone spinal anesthesia. Two additional patients with a history of recent spinal anesthesia demonstrated evidence of tuberculous meningitis. These results suggest that meningitis occurring in patients with a history of recent spinal anesthesia is often due to unusual or nosocomial organisms. Few data suggest that spinal or epidural anesthesia during bacteremia are risk factors for neuraxial infection. In the previously cited large studies, the number of patients who were febrile during the administration of the spinal or epidural anesthetic was not reported. A significant number of the patients in these studies underwent obstetric or urologic procedures, and it is therefore likely that at least some patients were bacteremic after (and perhaps during) needle or catheter placement. In a retrospective review by Horlocker et al.35 of 4,767 consecutive spinal anesthetics, there were two infectious complications noted. One patient, who developed a disc space infection following spinal anesthesia, was noted to have had a recent untreated episode of urosepsis. The second patient developed a paraspinal abscess 11 days following spinal anesthesia, performed after unsuccessful attempts at caudal blockade for suspected rectal fistula. Despite the apparent low risk of central nervous system infection following regional anesthesia, anesthesiologists have long considered sepsis to be a relative contraindication to the administration of spinal or epidural anesthesia. This impression is based largely on anecdotal reports and conflicting laboratory and clinical investigations.
Several studies have evaluated the risk factors for the development of epidural space infections in patients with indwelling epidural catheters. Darchy et al.36 studied 75 patients in an intensive care unit who were receiving epidural analgesia (median 4 days). Nine patients had local (catheter insertion site) infections, including four patients with epidural catheter (local inflammation with positive epidural catheter culture) infections, representing a frequency of 2.7 local (catheter insertion site) infections and 1.2 epidural infections per 100 days of epidural catheterization. Staphylococcus epidermidis was the most frequently cultured microorganism. All catheters were removed upon the appearance of discharge at the catheter insertion site, and antibiotic therapy was not specifically prescribed. The presence of both local erythema and discharge was associated with positive epidural catheter cultures. Concomitant infection at other sites, antibiotic therapy, and duration of indwelling epidural catheter were not significant risk factors for epidural infections. The authors recommended a meticulous daily inspection of the catheter insertion site and an immediate removal of the catheter if both erythema and local discharge are present. This recommendation precludes the use of dressings that obscure the catheter insertion site preventing inspection for signs of infection.
Controversy exists regarding the conditions under which a disconnected epidural catheter can be safely reconnected. In an in vitro investigation, epidural catheters containing a 5 µg/mL fentanyl solution were inoculated with S. aureus, E. coli, or P. aeruginosa37. Eight hours after catheter contamination, provided the fluid in the catheter remained static, no bacteria were detected more than 20 cm from the contaminated catheter hub. Vertical or horizontal positioning of the catheter during incubation did not affect bacterial advancement along the catheter, as long as the fluid was displaced distally <20 cm. However, if the fentanyl solution was allowed to drain and advance 33 cm, bacteria were found at the epidural end of the catheter 88 cm away. The advancement of bacteria by fluid displacement is clinically significant. In more than two-thirds of patients, fluid will drain by gravity into the epidural space in <1 hour after the discontinuation of an epidural infusion. The authors concluded that the interior of a disconnected epidural catheter would remain sterile for at least 8 hours if the fluid in the catheter remains static, and the catheter may be aseptically reconnected after the removal of the contaminated section. In addition, the presence of a meniscus >20 to 25 cm from the free end of a disconnected catheter may indicate the contamination of the catheter tip in the epidural space, and immediate catheter removal was recommended. Unfortunately, the authors did not evaluate the advancement of bacteria in epidural catheters filled with local anesthetic solutions or investigate the effect of a local anesthetic injected after bacterial inoculation and incubation.
Immunocompromise, due to either injection of epidural steroids or underlying disease processes, theoretically increases the risk of infection.38,39 In a case report, Strong39 described a 71-year-old man who developed S. aureus epidural abscess following the administration of methylprednisolone via thoracic epidural catheter (Fig. 5-2). An emergency decompressive laminectomy and antibiotic treatment resulted in a good outcome. Factors contributing to this patient’s epidural infection included an immunocompromised host (as suggested by the activation of a latent herpes infection), multiple catheter placements, and decreased immunologic response secondary to steroid administration. The use of an epidural in the presence of localized infection is somewhat controversial. Jakobsen et al.40 examined the records of 69 patients with localized infections who had a total of 120 epidural catheters placed, undergoing on average four epidural anesthetics with catheters left in place for a mean of 9 days. On 12 occasions, the catheter was removed due to local infection, no specific therapy was instituted, and the infection was resolved. There was one case of spondylitis, which was not apparently related to epidural catheterization. The retrospective nature of this study and the small number of patients limit the conclusions but suggest that placing an epidural catheter in a chronically infected patient may not be associated with a high risk of epidural infection.
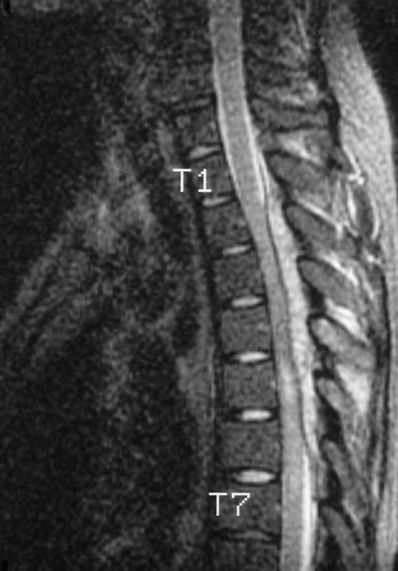
FIGURE 5-2. A thoracic epidural abscess is demonstrated on a magnetic resonance image in a patient who underwent thoracic epidural placement for the management of herpetic neuralgia. (From Finucane BT, ed. Complications of Regional Anesthesia. New York, NY: Churchill Livingstone, 1999:178, with permission.)



