Surgical site infections
Incisional
Superficial
Deep
Non-necrotizing SSTIs
Superficial infections (Impetigo, erysipelas, cellulitis)
Simple abscess, boils, and carbuncles
Complex abscesses
Necrotizing SSTIs (NSTIs)
Necrotizing cellulitis
Necrotizing fasciitis
Fournier’s gangrene
Necrotizing myositis
The first group includes surgical site infections (SSIs) of the soft tissues. They are considered a separate category of soft tissue infections. They are infections that occur after surgery within the surgical site at any depth from the skin itself and extend to the deepest cavity that remains after resection of an organ [2].
SSIs are classified into incisional and organ/space infections. Organ/space infections are not soft tissue infections. The incisional SSIs are further classified into superficial (skin and subcutaneous tissue) and deep infections (deep soft tissue–muscle and fascia).
Soft tissue non-surgical site infections are divided into non-necrotizing and necrotizing soft tissue infections. Non-necrotizing soft tissue infections include superficial infections, complex abscesses, and infections developing in damaged skin.
Necrotizing soft tissue infections (NSTIs) are life-threatening, invasive, soft tissue infections associated with widespread necrosis and systemic toxicity. They may involve dermal and subcutaneous components (necrotizing cellulitis), fascial component (necrotizing fasciitis (NF)), and muscular components (necrotizing myositis). Fournier’s gangrene is a progressive variant of necrotizing fasciitis involving the external genitalia and perineum.
NSTIs are also usually classified into three groups based on their bacterial pathogens initiating the infection: type 1 – poly-microbial, type 2 – mono-microbial pathogenic β-hemolytic Streptococci or community acquired methicillin-resistant Staphylococcus aureus CA-MRSA, and type 3 – mono-microbial secondary to a virulent gram-positive or gram-negative bacilli such as Clostridia, Vibrio, Aeromonas, Eikenella, and Bacillus species.
9.3 Principles of Treatment
9.3.1 Antimicrobial Therapy
The majority of SSTIs are caused by aerobic gram-positive cocci, such as Staphylococcus aureus and Streptococci. Aerobic gram-negative bacteria and anaerobes may occur in surgical site infections following surgery on the gastrointestinal, or genitourinary tract or soft tissue infections in the perineum or the genitourinary tract.
Among gram-positive bacteria, some strains of S. aureus and β-hemolytic Streptococci can produce toxins that may affect the severity of the soft tissue infection [1].
In the last years, emergence of Staphylococcus aureus resistant to penicillin has complicated antimicrobial therapy for SSTIs. Methicillin-resistant Staphylococcus aureus (MRSA) are usually acquired during exposure to healthcare facilities. However, in the last years, there has been an increase in MRSA infections presenting in the community (CA-MRSA) [3].
CA-MRSA strains are genetically and phenotypically distinct from hospital-acquired MRSA (HA-MRSA). They may be susceptible to a wider range of antistaphylococcal antimicrobials, and may produce the pathogenic Panton–Valentine leukocidin (PVL) toxin, a toxin that destroys white blood cells. The staphylococcal virulence factor [3] aggravates the severity of infection. Staphylococcal resistance to glycopeptides remains rare [4].
For empirical coverage of CA-MRSA in outpatients having SSTI, oral antibiotic options include clindamycin, trimethoprim-sulfamethoxazole (TMP-SMX), a tetracycline (doxycycline or minocycline), and linezolid. If coverage for both β-hemolytic Streptococci and CA-MRSA is required, options include clindamycin alone or TMP-SMX or a tetracycline in association with a β-lactam (e.g., amoxicillin) or linezolid alone [5].
For hospitalized patients with severe SSTI, in addition to surgical debridement and broad-spectrum antibiotics, empirical therapy for MRSA should be considered, pending culture data. Options include intravenous (IV) vancomycin 15–20 mg/kg/dose IV every 8–12 h, orally or IV linezolid 600 mg twice daily, daptomycin 4/6 mg/kg/dose IV once daily, IV or orally clindamycin 600 mg 3 times every day [5], tigecycline 100 mg IV loading dose, then 50 mg twice daily.
Staphylococcal resistance to glycopeptides remains rare, although rising minimal inhibitory concentrations (MICs) of glycopeptides may affect the efficacy of these antibiotics [1].
New antibiotics, such as dalbavancin and tedizolid, are extremely valuable additions to treatment options due to the convenient dosing regimen and the fact that there are fewer resistant organisms to these therapies at this time [6]. Telizolid is a second-generation oxazolidinone approved for the treatment of acute bacterial skin and skin structure infections (ABSSSIs). In a randomized, double-blind, phase 3, non-inferiority trial (ESTABLISH-2), once-daily tedizolid 200 mg for 6 days was non-inferior to twice-daily linezolid 600 mg for 10 days for treatment of patients with ABSSSIs [7].
9.3.2 Source Control
Source control represents a key component of success in the management of soft tissue infections [1]. Source control for SSTIs includes drainage of infected fluids, debridement of infected soft tissues, and removal of infected devices or foreign bodies.
In the setting of necrotizing infections, it must be prompt and aggressive in order to halt the progression of the infectious process.
9.4 Surgical Site Infections (SSIs)
The development of the SSIs depends on the contamination of the wound site during the surgical procedure. Surgical site infections may be caused by a variety of micro-organisms. In patients who have undergone clean operations, they are frequently caused by gram-positive organisms. In contrast, they may be caused by both gram-positive and gram-negative organisms in patients who had gastrointestinal or genitourinary surgery.
Treatment includes opening the incision. Antimicrobial therapy is required if source control is not complete or in immunocompromised patients. Broad-spectrum empiric antimicrobial therapy should be initially administered to cover potentially resistant pathogens [1].
9.5 Non-necrotizing SSTIs
Superficial infections are spread within the epidermis, dermis, and the subcutaneous tissue. They may be managed either by antibiotics alone or, in the case of a well-circumscribed abscess, by drainage alone.
Superficial infections present as impetigo, erysipelas, and cellulitis. They are usually caused by gram-positive bacteria, particularly Streptococci and S. aureus. Cellulitis is an acute bacterial infection of the dermis and the subcutaneous tissue that may cause local signs of inflammation, such as warmth, erythema, pain, lymphangitis, and systemic effects like fever and leukocytosis [1].
Therapy for these infections should include antibiotics active against Streptococci and S. aureus. A penicillinase-resistant penicillin is the drug of choice, although first-generation cephalosporin is an alternative. In the case of allergy to beta lactams, a fluoroquinolone regimen with either levofloxacin or moxifloxacin can be used.
Lack of clinical response could be due to resistant strains, or deeper active processes, such as necrotizing fasciitis or myonecrosis. In patients who become increasingly ill, deeper infections should be always suspected [1].
Incision and drainage is the primary treatment for a simple superficial abscess or a boil without the need for antibiotics [1].
Complicated abscesses can be caused by perineal or perianal infections, perirectal abscesses, diabetic foot or lower-extremity ulcerations, traumatic injuries, chronic cutaneous cysts, intravenous drug injection sites, gastrointestinal pathology with perforation, genitourinary pathology, animal bites, and pressure ulcers. Complicated skin and subcutaneous abscesses are typically well circumscribed and respond to incision and drainage with adjuvant antibiotic therapy.
Antimicrobial therapy is required in immunocompromised patients, if source control is incomplete, and for abscesses associated with significant cellulitis. The initiating pathogens differ according to the originating site. Aerobic gram-positive pathogens are isolated in most complicated abscesses. Depending on the origin, anaerobes, Enterobacteriaceae, and Clostridium spp. can be isolated.
9.6 Necrotizing Soft Tissue Infections (NSTIs)
NSTIs include necrotizing cellulitis, necrotizing fasciitis, necrotizing myositis, and Fournier’s gangrene. They are life-threatening aggressive soft tissue infections. Delay in treating these infections increases the risk of mortality.
NSTI can involve any part of the body but primarily the extremities, abdomen, and perineum [8].
NSTIs can be precipitated by various conditions like blunt or penetrating trauma, surgical site infection, burns, ulcers, abscess, improperly treated superficial infections, body piercing and tattooing, and even minor injury such as abrasions and insect bites. Occasionally, NSTI occurs without an identifiable cause [9].
Predisposing factors for necrotizing fasciitis include diabetes mellitus, immunosuppression, HIV, tuberculosis, and chickenpox. It is important to look for an underlying cause of immunosuppression, although it may not always be found [10, 11].
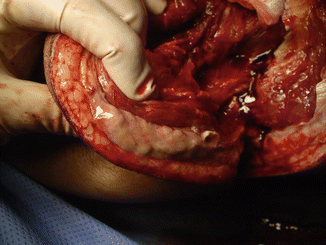
The mortality associated with NSTI is high ranging from 6 to 76 % [8]. Necrotizing cellulitis is usually similar to non-necrotizing cellulitis in bacterial etiology and pathogenesis but may be rapidly progressive and accompanied by significant systemic inflammatory changes (toxic shock syndrome). Necrotizing fasciitis (NF) involves the fascial planes overlying the muscle (Fig. 9.1). It is characterized by extensive, rapidly progressive necrosis involving the fascia and perifascial planes [1]. Fournier’s gangrene is a rapidly progressive, variant of necrotizing fasciitis involving the external genitalia and perineum. Because of the complexity of fascial planes, this infection may extend up to the abdominal wall, down into the perirectal and gluteal spaces, and, occasionally, into the retroperitoneum [1]. Necrotizing myositis is a rare infection of the muscle with local and systemic complications [1].