Fig. 12.1
Pre-operative computed tomography angiography (CTA) in a patient with RAAA. The white arrow depicts the site of aortic rupture in the anterior-left lateral wall. A blush of contrast media outside the aorta is evident, with voluminous intra-abdominal haematoma
Arteriovenous fistula (mostly aorto-caval) and aorto-enteric fistula are rare events; in this case, the rupture occurs into the venous system or into the bowel lumen, respectively.
12.1.2 Epidemiology and Mortality
In a recent Norwegian study, the adjusted incidence rate of RAAA is 11.0 per 100.000 per year and is by far higher in males (males: 17.6 per 100.00 per year vs. females: 4.7 per 100.00 per year) [3].
The risk of rupture mostly depends on the aneurysm diameter. According to recent data [4], cumulative yearly rupture rate is 3.5 % with diameter between 5.5 cm and 6 cm; 4.1 %, between 6.1 cm and 7 cm; and 6.3 %, >7 cm. Recent preliminary studies consider also additional parameters (geometrical AAA shape, female gender, arterial hypertension, smoking history, familiarity for AAA, intraluminal thrombus) to estimate individual AAA risk rupture [5, 6].
Patient died before reaching the hospital in about 45 % of cases and prior to undergo surgery in 25 % [7]. Among patients admitted to hospital for RAAA, intervention was not performed in 27 % of cases because of death, agonal status or patient’s decision [3]. Considering operated patients, 30-day/in-hospital mortality after surgery is about 46–48.5 % [8, 9]. Overall mortality of this condition is about 75 % [9].
12.1.3 Clinical Presentation and Diagnosis
A RAAA is characterised by the classical triad: pain, shock and pulsatile mass. Anterior rupture into the peritoneal cavity induces sudden abdominal or back pain. Collapse and death occur often before reaching the hospital [2]. In case of posterior aneurysm rupture, back pain is the main symptom; this condition may or not be associated with abdominal pain and hypotension. Retroperitoneal tissues may contain the rupture, so that patients may reach cure [2]. Unusual clinical presentations are transient lower limb paralysis, right hypochondrial pain, hydronephrosis, testicular pain or ecchymosis [2]. Pulsatile mass is not always palpable (i.e. in case of obese patients or severe hypotension).
Rupture of abdominal aortic aneurysm into the venous system (inferior vena cava, left renal vein, iliac veins) occur in about 3–4 % of RAAA with formation of arteriovenous fistulae. Adjunctive symptoms include abdominal bruit, dyspnoea, tachycardia, cyanosis, lower limb oedema, angina, palpitation, oliguria [2].
Rupture into the bowel creates an aorto-enteric fistula (<1 %) inducing haematemesis, melena and/or haematochezia [2]. Primary aorto-enteric fistulae are rare. Secondary fistulae must be considered in case of previous surgical aortic repair. The third and fourth portions of the duodenum are the most frequently involved.
Despite this frequent overt clinical presentation, misdiagnosis can occur [10]. Most common differential diagnoses are renal colic, myocardial infarction, colonic inflammation, gastrointestinal perforation [10]. Supporting imaging includes duplex ultrasound to confirm suspected diagnosis [1]. Computed tomography angiography (CTA) has nearly 100 % of accuracy [11] and provides important information for surgical intervention (lower renal artery, position of left renal vein, quality of the possible clamping zones) or endovascular procedure (length, diameter, calcification, tortuosity of iliac access and sealing zones).
12.1.4 Pre-operative Evaluation and Management
Smaller, peripheral hospitals are not equipped with a vascular surgery unit and, consequently, a rapid inter-hospital transfer is essential for a successful intervention. Geographical location of vascular surgery units should provide equity of access. Inter-hospital transfer is reported as an independent risk factor for mortality [12].
A standardised, multidisciplinary approach involving emergency specialists, vascular surgeons, anaesthesiologists and radiologists, and an efficient logistic (availability of duplex ultrasound and computed tomography, dedicated operating or hybrid room, intra-operative autotransfusion) are mandatory. Rapid imaging, trained physicians, nurses and other personnel, rapid availability of blood products and standard protocols are crucial for an adequate management [12].
Data on benefits from upfront transfusions are contradictory. Less recent publications [13–15] reported that, in case of haemorrhagic shock, massive fluid replacement could exacerbate the blood loss, inducing iatrogenic coagulopathy. By contrast, intravenous fluid transfusion <500 ml during pre-hospital phase with a target systolic blood pressure between 50 and 100 mmHg (permissive hypotension) may encourage clot formation. A recent publication states that aggressive transfusion before proximal clamping is a risk factor for death, independently from systolic blood pressure [16]. Data from prospective studies are lacking, and pre-operative transfusion policy varies among different centres.
According to the Guidelines of the European Society for Vascular Surgery [1], patients with a known abdominal aortic aneurysm, symptoms and shock should be immediately transferred to the operating room (after emergency ultrasound if available) [1]. In haemodynamically stable patients, urgent computed tomography angiography (CTA) is recommended. CTA has high accuracy in the diagnosis of RAAA providing data on aorto-iliac morphology [11], useful for decision-making processes. CTA can be performed in the majority of cases, and should be avoided only in severely unstable patients [10].
12.1.5 Operative Strategies: Open Repair
The intra-operative autotransfusion plays a role in reducing fresh frozen plasma use. Indeed, proportional greater use of autotransfusion is associated with lower mortality [17].
Median transperitoneal access is widely adopted, but left retroperitoneal approach (tenth intercostal space) is also described [18]. A rapid proximal control is required to stop the haemorrhage. It is crucial to preserve the integrity of the inferior vena cava, renal veins and oesophagus: this could be achieved with a subdiaphragmatic aortic cross-clamping. Then, it is suggested to rapidly isolate a more caudal segment of the aorta so the clamp can be moved distally, in a healthy portion of the vessel (preferably in an infrarenal position) for reducing the time of visceral ischaemia. In case of juxta- and infrarenal AAA, the preferred site of cross-clamping is supra- and infrarenal, respectively. Proximal control can be obtained also with intraluminal insertion of balloon catheter through the wall of the aneurysm [19] or by brachial [20] or femoral access [21].
Arterial reconstruction with aortoaortic tube or aortobiiliac bifurcated graft is performed depending on extension of aneurysmal disease (Figs. 12.2 and 12.3).
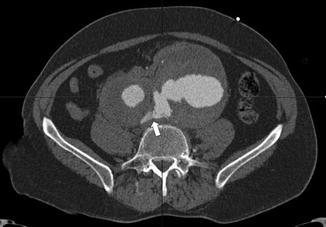
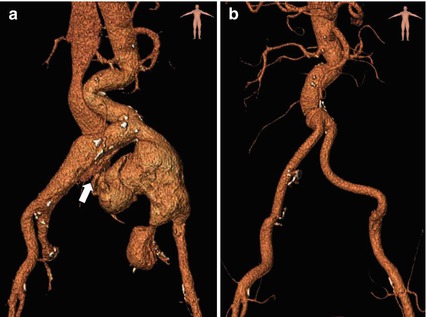

Fig. 12.2
Axial cut of a pre-operative CTA of a ruptured 11 cm aneurysm of the left common iliac artery, associated with a 5 cm aneurysm of the right common iliac artery. The white arrow indicates the rupture of the left common iliac artery aneurysm into the right iliac vein (arteriovenous fistula)

Fig. 12.3
Comparison between pre- and post-operative CTA through three-dimensional volume rendering reconstruction. (a) Pre-operative CTA showing the aneurysm rupture of the left common iliac artery into the venous system (white arrow). Visualisation of early passage of contrast media into the right iliac vein and the inferior vena cava. (b) Post-operative CTA after surgical aorto-bi-iliac grafting, with associated ligation of bilateral internal iliac arteries for hypogastric aneurysms
In case of tense abdomen and consequent difficult closure, open abdomen is left. After open surgical repair, primary closure of the abdomen is delayed in about 10 % [22] to avoid abdominal compartment syndrome with visceral organ dysfunctions.
12.1.6 Operative Strategies: Endovascular Repair
The first case of endovascular abdominal aortic aneurysm repair (EVAR) for RAAA treatment was reported in 1994 [23]. The lower invasiveness of the procedure compared with surgical treatment, and the possibility to perform it under local anaesthesia are the two characteristics that make EVAR an appealing option for RAAA.
However, aorto-iliac morphology must be fit for endovascular procedures in terms of access and sealing zones (both proximal and distal). An accurate pre-operative CTA evaluation is essential to plan the endovascular strategy and choose the most appropriate device. The rate of RAAA considered eligible for EVAR largely depends on personal endovascular skills and hospital organisation.
Aorto-uniiliac endograft deployment ensures a rapid control of bleeding. However, this procedure requires additional femoro-femoral crossover bypass and contralateral iliac occluder placement. Bifurcated grafts are largely used in emergency, but an adequate stock of endografts is necessary which is unlikely to be available in all centres.
In case of haemodynamic instability, the occlusion of the supraceliac aorta with an inflatable balloon leads to haemorrhage control.
12.1.7 Post-operative Management and Complications
The post-operative development of intra-abdominal hypertension and abdominal compartment syndrome (ACS) is one of the most frequent complications after surgical or endovascular treatment of RAAA. ACS is defined as intra-abdominal pressure >20 mmHg associated with new organ dysfunction or failure [22]. ACS can also be present with an intra-abdominal pressure <20 mmHg in case of hypotension (mean arterial pressure <60 mmHg). Incidence of ACS is about 20 % after both surgical and endovascular treatment [24, 25]. Medical support consists of neuromuscolar blockade, positive end-expiratory pressure, albumin and diuretics [22]. Some Authors [26] described CT-guided puncture of the abdominal haematoma with drainage insertion and injection of tissue plasminogen activation to facilitate clot dissolution and abdominal decompression. Decompression laparotomy is still often required. Temporary abdomen closure is needed to avoid bowel fistula formation and is obtained by meshes or patches and, sometimes, vacuum therapy [22].
Peri-operative myocardial ischaemia occurs in about 10–15 % of treated RAAA [27, 28] without statistical differences between open and endovascular treatments.
Pneumonia and respiratory complications are reported in about 15–25 % of cases, independently from strategy of RAAA treatment [27, 28].
Worsening of renal function after surgical procedure for RAAA is a common complication [29] caused by hypovolaemic shock, reperfusion and cross-clamping. During EVAR, iodinated contrast agent can cause nephrotoxicity. Severe acute renal failure occurred in 36 % of patients treated for RAAA with higher percentage after surgical repair (43 % after open repair vs. 26 % after EVAR) [30].
Bowel ischaemia is a high-mortality complication, that burden open surgery and endovascular treatment in 42 % and 23 % of cases, respectively [31, 32]. The pathogenesis of intestinal ischemia is multifactorial: hypotension, cross-clamping, embolisation, exclusion of hypogastric arteries or inferior mesenteric artery, reperfusion injury [32]. Sigmoidoscopy is recommended during the post-operative period for early diagnosis [32].
12.1.8 Outcomes
Comparison between surgical and endovascular treatment of RAAA has been one of the most discussed topics in the last years. Data from several retrospective studies and randomised trials has been largely analysed [33–35] with risk of bias for clinical and anatomical characteristics and different enrolment criteria. A recent meta-analysis, analysing three randomised trials [36], showed no statistically significant difference in terms of 30- and 90-day mortality according to the surgical approach.
Even considering 1-year mortality, mortality was overlapping between surgical and endovascular intervention (38.6 % in EVAR group vs. 42.8 % in open repair group) [36].
12.2 Major Abdominal Vascular Injuries
Abdominal vascular injuries after a penetrating or blunt trauma are often lethal at the scene and never reach medical care [37]. Overall, these traumas are characterised by high mortality rates that can exceed 40 %, according to the most recent literature [38–41]. A rapid transfer to a trauma centre with adequate pre-hospital and emergency department treatment, a good medical judgement and an early surgical intervention are of vital importance to obtain a positive outcome.
12.2.1 Anatomy, Mechanism of Injury and Epidemiology
The abdomen is conventionally divided into three topographic zones, which not only define the anatomic area of the lesion, but also drive the therapeutic decisions [42]:
Zone 1: it includes the midline retroperitoneum from the diaphragm to the sacral promontory. This zone is in turn subdivided according to the possible surgical approach as follows:
Supramesocolic area: suprarenal aorta with its major branches, suprarenal inferior vena cava (IVC), superior mesenteric vein.
Inframesocolic area: infrarenal aorta and IVC, superior mesenteric artery distal to the middle colic artery, inferior mesenteric artery.
Zone 2: it includes the right and left paracolic gutters and the renal vessels.
Zone 3: it includes the pelvic retroperitoneum below the sacral promontory, with the iliac vessels and the presacral venous plexus.
Portal vein, hepatic artery and retrohepatic IVC lesions are considered separately.
Abdominal traumas are distinguished in penetrating or blunt, according to the mechanism of injury. Penetrating trauma is accountable for most abdominal vascular lesions (90–95 % of patients [43, 44]) and is generally attributable to knife and gunshot injuries [45]. All abdominal vessels can be involved, and all types of vascular damage can occur (tearing, transection, intimal dissection, thrombosis, pseudoaneurysm, arteriovenous fistula). High-velocity bullets can also determine vascular lesions by means of the shock wave and transient cavitation, leading – usually – to acute thrombosis of nearby arteries [46]. Rare causes of penetrating aortic trauma include misplacement of spinal fixation screws, and laceration from spinal fractures [47, 48].
Blunt abdominal traumas are mostly associated with high-speed motor vehicle collisions (steering-wheel, seat-belt and lap-belt injuries), and vascular injuries may be secondary to:
- 1.
Compression/direct anteroposterior crushing
- 2.
Rapid deceleration (avulsion or intimal tear and subsequent thrombosis)
- 3.
Direct laceration by a bone fragment dislocation
Blunt abdominal aortic injuries represent about 4–5 % of blunt aortic injuries [49].
In 2009, Azizzadeh et al. [50] proposed a classification to guide management of aortic injuries based on imaging appearances:
Grade I: intimal tear
Grade II: intramural haematoma
Grade III: pseudoaneurysm (the most common to present clinically)
Grade IV: rupture
Abdominal arterial and venous lesions occur with the same incidence [42]. Most frequently injured vessel are the IVC (25 % of injuries in a large series of abdominal vascular traumas [43]), followed by the aorta (21 %), the iliac arteries and veins, the superior mesenteric vein and the superior mesenteric artery. The majority of the patients have more than one vascular lesion.
12.2.2 Clinical Presentation and Diagnosis
The clinical presentation depends on many variables:
The mechanism of injury (penetrating vs. blunt)
The injured vessel
The size and type of the lesion (i.e. the presence of a retroperitoneal hematoma vs. a free rupture)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







