Fig. 19.1
The denser the material is the more it reflects the sonographic waves. Fluid (like blood) transmits ultrasound waves and have minimum waves reflected back. This yields a black “anechogenic” image. Soft tissues (like the liver) yield different grey colour scales. Fibrous tissue will be white without a shadow (like the diaphragm). Stones yield white images (hyperechoic) with a shadow behind them (Reproduced with permission from Abu-Zidan et al. [7])
There are different ways in which an ultrasound image can be modified including the frequency of the wave, the size and shape of the ultrasound probe, and the timing in which the waves are emitted [6, 7]. Abdominal examination in obese adults and deeper structures needs lower frequency probes (2–5 MHz) having deep penetration. In contrast, probes of high frequency (10–12 MHz) have better resolution and less depth of penetration. They should be used for superficial structures [2]. Ultrasound waves are generated perpendicular to the surface of the probe. Bending the surface of the probe will widen its field and reduce its resolution at depth (convex array transducers). Flat probes will give a rectangular image with good resolution in the whole image (linear array transducers). Small print ultrasound probes are needed for imaging thoracic structures using the sonographic windows between the ribs [8].
19.3 Detection of Intra-peritoneal and Chest Fluid
Ultrasound is very sensitive in detecting fluid in the peritoneum and pleura [9]. Detection of intra-peritoneal and pleural fluid is a simple skill that can be easily learnt by surgeons, or emergency physicians, if they were properly trained [10, 11]. Fresh blood is generally hypoechoic on ultrasound. Nevertheless, clotted blood will be echogenic and can be missed. Ultrasonography cannot differentiate between different types of fluid including blood, urine, bile, lymph, transudate, or exudate [12]. The nature of the fluid can be known if correlated with the clinical picture or if tapped. Intra-peritoneal fluid in a hypotensive patient is most probably blood.
Currently, ultrasonography is the modality of choice in detection of haemoperitoneum in blunt trauma patients (Fig. 19.2). Ultrasound has replaced the diagnostic peritoneal lavage in the majority of patients. Performing a secondary ultrasound examination, even in stable patients, increases the sensitivity of ultrasound in detecting intra-peritoneal free fluid [13].
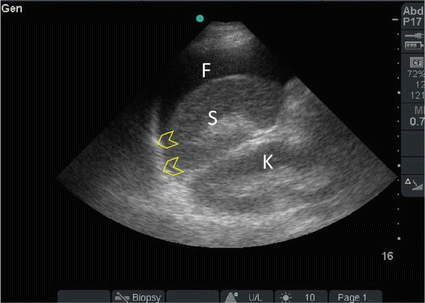

Fig. 19.2
A 36-year-old front seat male passenger, who was not wearing a seatbelt, was involved with a front impact collision and was ejected through the front wind screen. He was haemodynamically stable but had tenderness and guarding all over the abdomen. Surgeon-performed POC ultrasound of the abdomen showed free intra-peritoneal fluid (F) between the spleen (S) and diaphragm (arrowheads). The patient had a laparotomy. He had extensive mesenteric tears with small bowel and sigmoid colon injuries without solid organ injuries (Sonographic study was performed by Professor Fikri Abu-Zidan, Department of Surgery, Al-Ain Hospital, Al-Ain, UAE)
Intra-peritoneal fluid is likely to collect in the most dependent areas. These include hepatorenal space (Morison’s pouch), left subphrenic space or splenorenal recess, and pelvis (retrouterine pouch or retrovesical pouch) [14, 15]. The site where maximum fluid is collected depends on the position of the patient. Fluid will accumulate in the hypochondria in the Trendelenburg position while it will accumulate in the pelvis in the anti-Trendelenburg position. We usually examine the patient in the horizontal position because that is required for measuring the IVC diameter during resuscitation. Fluid in the pericardial sac can be detected in the subxiphoid or parasternal views while pleural fluid can be detected in the coronal sonographic view of the hypochondria [16].
19.4 Echocardiography
Echocardiography is a very useful diagnostic modality in life-threatening conditions. It can also guide interventional procedures like aspiration of the pericardial effusion. We usually use a small print probe having 2–5 MHz. We start always by the subxiphoid and four-chamber apical views [17, 18]. These are usually enough to get the information needed in the acute care setting to make critical decisions. Severe obesity, distended stomach, subcutaneous emphysema, lung inflation due to chronic obstructive airway disease, or chest deformity may limit the access to these windows. Long parasternal and short parasternal views are used when other windows are difficult to obtain.
As acute care surgeons, we always keep the marker of the probe to the right (contrary to cardiologist who keep it to the left). This is important because echocardiography will be performed as an extension of the abdominal examination especially in shocked patients. The setting of the machine will not be changed, and the right ventricle will be on the right side of the screen. The subxiphoid four-chamber view can be obtained by placing the probe left to xiphoid process with the beam directed towards the left shoulder. The apical four-chamber view can be obtained by placing the probe on the heart apex beat (fifth intercostal space at midclavicular line). The beam should be directed towards the right shoulder [17, 18].
In focused echocardiography, simple questions are usually answered. These include: (1) Are the heart ventricles contracting properly? (2) Is there pericardial fluid? (3) What is the volume of each ventricle: Is it enlarged, normal, or reduced in volume. The answers to these simple questions can lead to very important information.
The subxiphoid view is the best to diagnose pericardial effusion around the right ventricle and the right atrium as it shows a hypoechoic area between the liver and the heart [19] (Fig. 19.3). Pericardial effusion on the left side of the heart is easier to differentiate on long parasternal view as it will show as a hypoechoic area anterior to the aorta compared to pleural effusion which will be behind the aorta. The pressure of the pericardial fluid on the heart causes a tamponade effect. Compression of the right ventricle is easier to observe during early diastole while compression of the right atrium is easier to observe during late diastole. IVC diameter will increase and will lose the inspiratory collapse. Occasionally, you may see a thrombus in the ventricle or atrium.
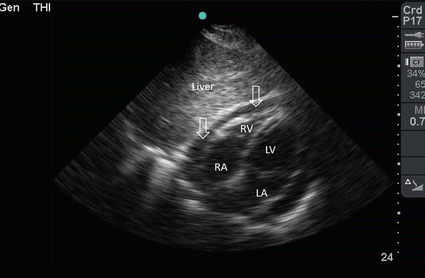

Fig. 19.3
A 36-year-old male driver who was involved with a front impact road traffic collision. He had chest trauma treated by bilateral chest tubes in another hospital, then transferred to our hospital 2 weeks later. He was haemodynamically stable. POC ultrasound of the heart using a subxiphoid approach showed moderate amount of pericardiac fluid (arrows) not affecting the function of the heart. The patient was treated conservatively. RA right atrium, RV right ventricle, LV left ventricle, LA left atrium (Sonographic study was performed by Professor Fikri Abu-Zidan, Department of Surgery, Al-Ain Hospital, Al-Ain, UAE)
Massive pulmonary embolism will increase the resistance to the flow from the right ventricle. The volume of the right ventricle will be enlarged and the interventricular septum will be pushed towards the left ventricle [19] (Fig. 19.4). This changes the shape of the right ventricle in the short axis view from a C shape to a D shape. In severe sepsis without hypovolemia, the volume of both ventricles will be dilated and their contractility will be reduced due to the cardiac inhibition caused by endotoxins. In severe bleeding or hypovolemia, the heart will be tachycardic and the ventricles will be empty [18]. Echocardiography is very useful during resuscitation as it can accurately diagnose the pulseless electrical activity when there is no contractility of the heart.
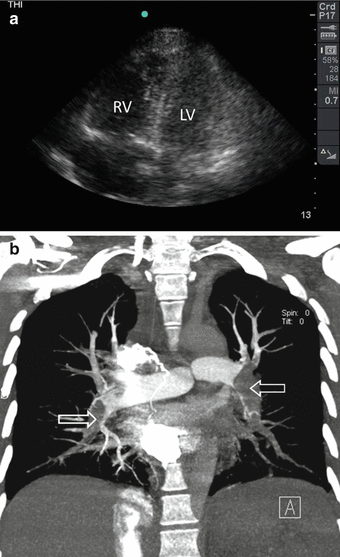

Fig. 19.4
A 35-year-old female developed syncope, shortness of breath, and shock following a 9 h air flight. POC ultrasound shows a dilated right ventricle (RV), which was larger than the left ventricle (LV). The RV was not properly contracting (a). CT angio showed the presence of massive bilateral pulmonary embolism (arrows) (b) (Sonographic study was performed by Professor Fikri Abu-Zidan, Department of Surgery, Al-Ain Hospital, Al-Ain, UAE)
19.5 Measuring IVC Diameter
The value of measuring the IVC diameter in shocked patients is debatable [20, 21]. We advocate imaging the IVC directly between the ribs at the right midclavicular line at the lower chest wall so as to locate the IVC in its longitudinal section. For this we use a small print convex array probe having a frequency of 3–5 MHz. We use the longitudinal B mode to evaluate the gross collapsibility of the IVC while we use the M mode to calculate the percentage of changes in IVC diameter both longitudinally and transversely [22].
The technique to measure the IVC should be standardized. The ultrasound beam should be vertical to the IVC section. Common pitfalls encountered in measuring the IVC diameter include sectioning the IVC peripherally or obliquely (Fig. 19.5). We found that relative visual changes of the IVC are more useful than absolute calculated numbers. Combining these two approaches will increase the value of IVC measurement in evaluating shocked patients. If the operator defines the area in which he repeatedly measures the IVC for a specific patient, then the results will be more reliable.
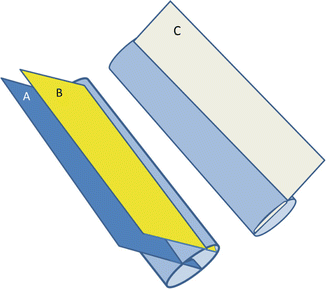

Fig. 19.5
A figure demonstrating the technique to measure the IVC diameter longitudinally. The ultrasound cross section should be vertical to the IVC (A). Common pitfalls in measurement include measuring the IVC peripherally (B) or obliquely (C) (Reproduced with permission from Abu-Zidan [22])
Common pitfalls that falsely increase the IVC diameter include increased right atrial pressure during artificial ventilation, severe sepsis without hypovolemia causing increased resistance of the pulmonary circulation (Fig. 19.6), or by pulmonary embolism [23]. In contrast, increased intra-abdominal pressure in abdominal compartment syndrome will cause direct pressure on the IVC and give false small IVC diameter [24]. The measurement of IVC diameter should be correlated with the findings of echocardiography and the clinical picture as a whole.
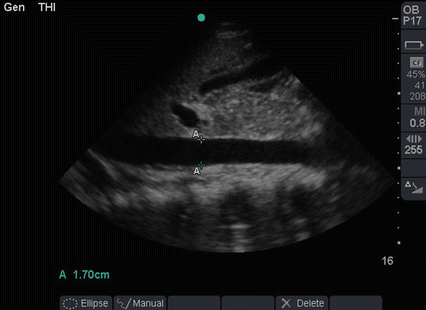

Fig. 19.6
A 30-year-old 10 weeks pregnant women presented with fever, abdominal pain, and vaginal discharge of 7 days duration. When seen in the ICU, she was ventilated, having a blood pressure of 50/30 mmHg and a pulse of 130 bpm. Her abdomen was distended. POC ultrasound showed an intrauterine dead fetus and minimal intra-peritoneal fluid. Both cardiac ventricles were dilated and weakly contracting. The IVC was grossly dilated (1.7 cm) which ruled out hypovolemia. The POC ultrasound were highly suggestive of septic shock caused by infected abortion. The uterus was evacuated from its contents under general anaesthesia. The patient survived and went home in good condition (Sonographic study was performed by Professor Fikri Abu-Zidan, Department of Surgery, Al-Ain Hospital, Al-Ain, UAE)
19.6 Lung Ultrasound
Understanding the reverberation artefact is central for the interpretation of lung ultrasound [6, 7]. This artefact is the normal pattern of the lung. Both visceral and peripheral pleura are fibrous tissue that are white in colour. Since they are moving during inspiration, pleural sliding will be clearly demonstrated. The pleura are strong ultrasound reflectors. When ultrasound is emitted from the ultrasound probe, it will be reflected from the pleura. Nevertheless, some waves repeatedly bounce between the ultrasound probe and the pleura [6, 7]. As the reflected waves are gradually picked up by the ultrasound probe, the bouncing waves become gradually less. The ultrasound machine receives these waves as parallel white horizontal lines with gradual decrease in density and equal distance between them. These are called A lines (Fig. 19.7).
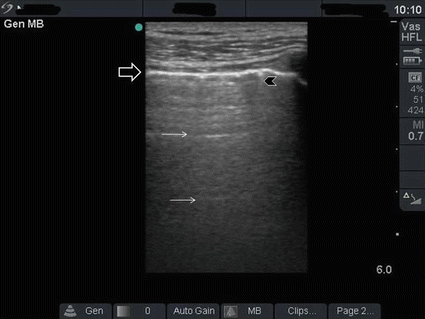

Fig. 19.7
Reverberation artefact of the lung occurs as ultrasound waves bounce between the transducer and the pleura. The pleura is shown as a hyperdense white line (black arrow). The reverberation lines (white arrows) represent repetition of the pleural line. The distance between these lines are equal. A comet tail artefact is also shown (black arrowhead) (Reproduced with permission from Abu-Zidan et al. [7])
Abolished lung sliding and A lines can been seen in any lung that does not move [25]. This can be possibly a pneumothorax or alternatively a lung collapse caused by a plug or contralateral tracheal intubation. Presence of lung sliding rules out pneumothorax [25]. Nevertheless, ultrasound will only inform us that the lung is not moving and this should be correlated with the clinical picture to know the cause. The only accepted pathognomonic sign for a pneumothorax is the presence of the lung point (the dynamic change of the edge of the lung sliding) [25].
The air in the lung is very reflective and does not permit ultrasound waves to be transmitted. Nevertheless, when there are some alveoli with scattered fluid in the alveolus or around it, then shiny vertical lines that pass through the whole ultrasound image are present. These are called B lines [25]. Any fluid may cause these lines including pulmonary oedema, pneumonia, aspiration, or lung contusion. The number of B lines and extent involved in the chest area will reflect the severity of the pathology. The B lines represent white areas on the chest X-ray. Accordingly, both lungs can be mapped by ultrasound (Fig. 19.8). Complete collapse of the lung will be clearly seen on ultrasound similar to a liver tissue because there is no or little air in the alveoli.
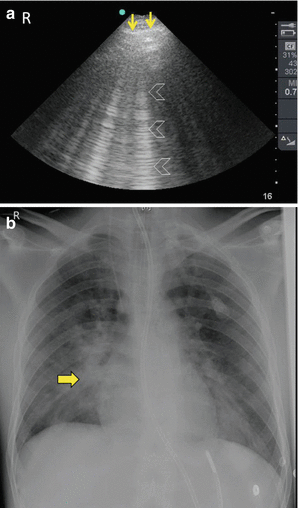

Fig. 19.8
A 26-year-old man who fell from 3 m high. He sustained open fracture of the left tibia and spine fracture. He suddenly developed hypoxia in the ward without a clear cause and was transferred to the ICU. Pulmonary embolism was suspected. POC ultrasound of the right lung using a portable ultrasound machine and a small print convex array probe with a frequency of 3–5 MHz demonstrated extensive B lines in the right lung (arrowheads) indicating the source of hypoxia (a). Chest X-ray confirmed the findings (yellow arrow) (b). The patient was successfully treated for aspiration of the right lung
19.7 Scanning the Aorta
The aorta should be surveyed by ultrasound in any patient having unexplained shock. Sonography is recommended as an accurate diagnostic test in that situation [26]. Abdominal probe having a frequency of 2.5–3.5 MHz is used [26, 27]. The aorta is located to the left of the midline. The aorta will be pulsatile and the coeliac axis and superior mesenteric arteries will be seen stemming from the aorta anteriorly in the longitudinal section. Turn the probe 90° with the marker pointing to the right and see the transverse section of the aorta. Scan the aorta starting from the epigastrium and follow it distally till the bifurcation where the two common iliac arteries are seen.
The outer diameter should be included in the measurement. A diameter larger than 3 cm or more than 1.5 times of the proximal uninvolved diameter of the aorta should be considered abnormal [26, 28]. A thrombus may be seen within the aortic lumen. Flow or Colour Doppler can be used to study the flow within the aneurysm. Finding free intra-peritoneal fluid is very serious as it may indicate a ruptured aortic aneurysm. It may be difficult to visualize the abdominal aorta from the anterior abdominal approach because of presence of gas or obesity. Gentle compression may be needed to move the bowel gas away from the view. Try to visualize the aorta from the left lateral side through the retroperitonium if you encounter difficulty while using the anterior approach [27] (Fig. 19.9).
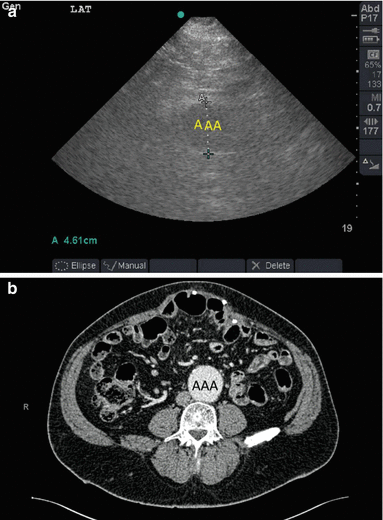

Fig. 19.9




A 65-year-old obese male presented to the Emergency Department complaining of a painful upper abdominal incisional hernia. The hernia was reducible. A pulsatile mass was felt above the umbilicus. Scanning of the aorta by a portable ultrasound machine using a small print convex array probe with a frequency of 3–5 MHz and a lateral abdominal approach demonstrated an abdominal aortic aneurysm (AAA) having a diameter of 4.61 cm (a). CT with intravenous contrast has confirmed the diagnosis (b) (Sonographic study was performed by Professor Fikri Abu-Zidan, Department of Surgery, Al-Ain Hospital, Al-Ain, UAE)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







