Epistaxis
Systemic causes
Vascular disorders (hypertension, Rendu-Osler-Weber disease)
Congenital or acquired heart diseases
Diabetes
Liver failure
Kidney diseases
Viral and bacterial infectious diseases
Antiplatelet and anticoagulant therapies
Vitamin deficiencies (vit. C, vit. K)
Hemopathies (von Willebrand disease, hemophilia)
Endocrine disorders
Autoimmune disorders (Wegener disease, etc.)
Barotraumatisms
Paraneoplastic syndromes
Local causes
Nasal surgery (FESS, septoplasty, etc.)
Traumatisms
Neoplasms
Nasal foreign bodies
Nasal septum varicose veins
Acute rhinitis
Although epistaxis usually represents a benign symptom, it can quickly turn into a life-threatening condition. As a matter of fact, the majority of cases are controlled with common first-line intervention such as electrical cautery, the application of hemostatic agents, or anterior nasal packing, but there is a subset of patients who continue to bleed and require more aggressive therapy. It is therefore important to distinguish anterior nosebleeds from posterior ones.
The majority of epistaxis originates from the anterior nasal septum (locus Valsalvae) and is classified as anterior epistaxis. The minority of cases bleeds beyond a classic anterior rhinoscopy, mainly from the posterior lateral nasal wall and the posterior nasal septum, and is so classified as posterior epistaxis.
The aim of this chapter is to focus on the intractable epistaxis, whose origin is most commonly the sphenopalatine artery (SPA). Epistaxis from the AEA more rarely occurs in association with midface trauma or iatrogenic injury during endoscopic sinus surgery [1].
A thorough knowledge of the anatomy is necessary to optimize the surgical treatment, so we will follow a brief but comprehensive description of the vascular and surgical anatomy of the nasal cavity.
6.2 Nasal Vascular Anatomy
The frequency of nosebleeds is linked to the richness of the nasal vascular supply, which is provided from the external carotid artery system through maxillary and facial arteries and from the internal carotid artery system through the ophtalmic artery with its anterior and posterior ethmoidal branches [3].
6.2.1 External Carotid Artery System (ECA)
6.2.1.1 Maxillary Artery (Internal Maxillary Artery: IMA)
The maxillary artery, a terminal branch of the external carotid artery along with the superficial temporal artery, enters the pterygopalatine fossa through the pterygomaxillary fissure, where it gives life to the descending palatine artery and the pterygopalatine artery that supplies the mucosa of nasopharynx and choanae. Once passing through the sphenopalatine foramen, it takes the name of sphenopalatine artery (Fig. 6.1), its terminal branch, and then it splits into medial and lateral branches. The medial one, or posteroseptal nasal artery (Fig. 6.2), emerges from upper portion of the sphenopalatine foramen and gives rise to a branching for the upper turbinate, and then it goes inward to reach the septum. Then it flows by a groove excavated along the ethmoid-chondral-vomerian suture to enter the anterior palatine canal and join the septal branch of the superior labial artery. Along its course, it also provides some septal branches.
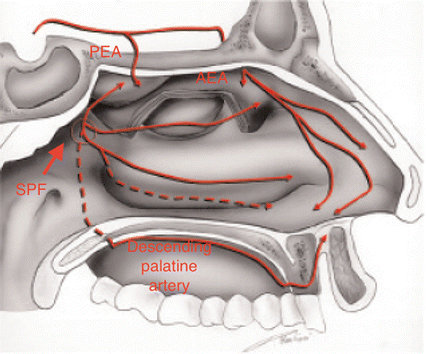
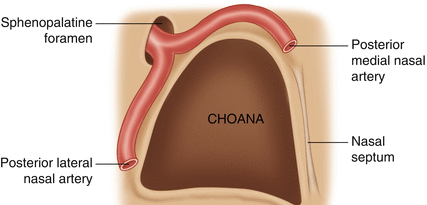

Fig. 6.1
Sphenopalatine foramen (thick arrow) and the lateral nasal wall supply by the sphenopalatine artery

Fig. 6.2
SPA splitting into the posteroseptal nasal artery and the posterior lateral nasal artery
The lateral one, or posterolateral nasal artery, emerges from the lower portion of the sphenopalatine foramen to provide the middle nasal artery for the region of the middle meatus and the inferior nasal artery that goes to the inferior turbinate.
6.2.1.2 Facial Artery
The facial artery provides the superior labial artery at the level of the upper lip and then it anastomoses with the contralateral one to form the superior coronary arcade and becomes angular artery rising up along the nasolabial fold. From the superior coronary arcade arises the septal branch of the superior labial artery that flows along the anterior-inferior portion of the septum to provide septal and vestibular branches. The angular artery supplies the lateral face of the pyramid.
6.2.1.3 Internal Carotid Artery System (ICA)
The ophthalmic artery originates from the internal carotid artery at the level of the anterior clinoid process of the sphenoid bone and enters the orbit through the optic canal along with the optic nerve. It provides nasal vascular supply through anterior and posterior ethmoidal arteries. Its other collateral branches are the central retinal artery, ciliary arteries, lateral palpebral arteries, and the supraorbital artery.
6.2.1.4 Anterior Ethmoidal Artery (AEA)
The anterior ethmoidal artery reaches the anterior ethmoid foramen whose orbital orifice is located approximately 15 mm from the orbital edge. The insertion of the anterior superior ethmoid bulla is an important landmark both for the frontal recess and the AEA, which runs on the ethmoid roof and is separated from the frontal recess by the first ethmoid foveola (Fig. 6.3); the artery is present on its dorsal edge [4]. After crossing the ethmoid roof passing through the ethmoidal lateral lamella, AEA enters the cranial cavity giving rise to a nasal branching that reaches the anterior superior part of the nasal cavity through the cribriform plate.
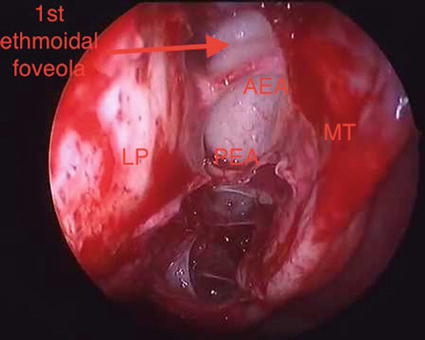

Fig. 6.3
Right nasal fossa: LP lamina papiracea, first ethmoidal foveola, MT middle turbinate, AEA anterior ethmoidal artery and PEA posterior ethmoidal artery
From this nasal branching originate the lateral and medial branches. The lateral ones supply the anterosuperior part of the lateral nasal wall, the anterior ethmoid, and the frontal sinus. The medial ones supply the anterior superior portion of the septum. The terminal tract of the anterior ethmoid artery then gives rise to a meningeal branch in front of the olfactory shower level [3, 5].
6.2.1.5 Posterior Ethmoidal Artery (PEA)
The posterior ethmoidal artery is much smaller than the anterior one, it runs on the posterior ethmoid roof in a very thick bony canal, so its dehiscence or injury is very rare [5]. PEA reaches the posterior ethmoid foramen in order to supply posterior ethmoidal cells, the posterior-superior portion of the lateral nasal wall, and the septum at the level of the olfactory shower. The relatively high frequency of posterior ethmoidal artery agenesia (12.5 % of cases) should be also emphasized.
These two arterious systems are abundantly anastomosed, as briefly described below [3]:
At the level of the septum:
Anastomosis on the anterior nasal septum where the locus Valsalvae (or Little’s area), which is supplied by Kiesselbach’s plexus, is formed by the confluence of the sphenopalatine artery (SPA – terminal branch of the maxillary artery), the greater palatine artery (from the maxillary artery), medial branches of anterior and posterior ethmoidal arteries (from the ophthalmic artery), and the septal branch of the superior labial artery (from the facial artery).
Anastomosis between posterior ethmoidal artery and branches from the sphenopalatine artery.
On the superior lateral wall: anastomosis between ethmoidal arteries and upper turbinate artery.
6.2.2 Venous System
Particularly represented at the level of the turbinates as cavernous tissue, it is formed by two different routes of drainage, communicating with each other, that refer to the internal jugular vein and the cavernous sinus, as follows [2–4]:
Superiorly, the blood is drained from the superior ophthalmic vein, tributary of the cavernous sinus, through the ethmoidal veins; some venous branches can perforate the cribriform plate to flow into the veins of the frontal lobe of the brain.
On the posterior side, after passing through the sphenopalatine foramen, veins flow into the pterygoid plexus surrounding the pterygoid muscles and from which maxillary veins arise; at first they flow into the posterior facial vein (or retromandibular vein) and then into the internal jugular vein. The most important connection between pterygoid plexus and cavernous sinus, mainly through the oval foramen, is the “Vesalius vein”; pterygoid plexus is also connected with the facial vein (via the deep facial vein) and the pharyngeal plexus.
There is a rich venous vascular network called “Woodruff plexus” at the level of the rhinopharynx, on the posterior side of middle and inferior meati.
Some veins flows anteriorly into the facial vein.
6.2.3 Surgical Anatomy of the SPA
Just as described above, the SPA is a terminal branch of the IMA and enters the nasal cavity at the level of the posterior attachment of the middle turbinate through the sphenoplatanine foramen (SPF). The SPF sets a communication between the pterygopalatine space, laterally, and the nasal cavity, medially. It is formed by the sphenoid bone body that closes superiorly the sphenopalatine incisure of the palatine bone. This portion is in relationship anteriorly with the palatine orbital process, posteriorly with the palatine sphenoidal process, and inferiorly with the superior border of the palatine perpendicular lamina.
Three classes of anatomical variability of the SPF localization have been identified. Class I SPF (35 % of cases) is identified completely above the posterior projection of the middle turbinate. Class II SPF (56 % of cases) has its lower border below the crista ethmoidalis. Crista ethmoidalis or ethmoidal process of the palatine bone is a slight relief of the medial face of its perpendicular lamina.
Class III SPF (9 % of cases) is characterized by two distinct foramina: the superior one in the upper meatus and the inferior one in the middle meatus.
But according to Lee et al. [6], SPF in located in 90 % of cases in the upper meatus, superiorly to the crista ethmoidalis.
In all cases, crista ethmoidalis can be considered as an important surgical landmark for its relationship with the SPF.
Furthermore it has been founded that in most cases, the SPA splitting in three or four branches occurs in the infratemporal fossa before its entrance in the nasal cavity. This situation has important clinical and surgical consequences such as the possibility of a selective ligation of the only bleeding vessel, so sparing the rest of the vascular supply but also the vitality of turbinates. Indeed some cases of necrosis of the turbinates after SPA ligation are well described in literature.
Although several anatomic variations are described in the literature, all studies indicate that the SPF is usually located posteriorly at the junction of the middle and superior meati. The ethmoid crest (or crista ethmoidalis) is an important surgical landmark, because it is always present and is located anteriorly to the SPF in 98 % of cases [4].
6.2.4 Surgical Anatomy of the AEA and PEA
To sum up, the AEA branches off medially the orbital cavity and supplies the superior lateral nasal wall and septum after reaching the ethmoid skull base. Externally, the AEA can be identified about 2 cm posterior to the lacrimal crest, in the space between the orbital periosteum and lamina papyracea. Endonasally, it can be located either at the ethmoid skull base attachment of the ethmoid bulla lamella or just posterior to this site, along the posterior wall of the frontal recess.
Critical aspects of the surgical anatomy of the ethmoidal arteries concern the location variability on the coronal plane, related to the ethmoidal roof and to lateral lamella, and on the sagittal plane, because of the relationship with the ethmoidal lamellae.
On the sagittal plane, the AEA is located in most cases between the ethmoid bulla lamella and middle turbinate lamella. Simmen et al. have identified the AEA at the average distance of 11 mm from the posterior wall of the frontal recess.
On the coronal plane, another key element is the relationship between the AEA and the ethmoidal roof. Recent cadaver studies indicate that AEA runs in a bony arterial mesentery in 35.5 % of cases, with an average distance of 3.5 mm from the skull base, while it is amenable to surgical ligation in about 20 % of cases. Patients with an AEA located in a mesentery can be identified on preoperative CT and they tend to have longer lateral lamella (Keros type 2 or 3) and a high ethmoid skull base [1].
Other aspects concern the possibilities of dehiscence of their bony canals and the different spatial orientation of their pathways.
Some authors have identified real spatial coordinates to reach the AEA: the optical endoscopic stretched between the dome of the alar cartilage and the axilla of the middle turbinate is a real “pointer” oriented toward the AEA in the fovea ethmoidalis [4].
6.2.5 General Management of Epistaxis
The first priority during intractable epistaxis is to slow or trying to control the active hemorrhage with temporizing packing in order to occlude the posterior choanae and avoid the blood inhalation through the upper airway. In the past few years, the classic posterior nasal packing has been giving the way to anterior-posterior balloon combo packs such as Bivona® or a Foley® catheter combined with anterior packing.
Once the active bleeding is controlled, a meticulous medical history investigation is necessary to identify critical medical factors and correct them if possible.
Recurrent episodes of epistaxis in elderly patients in anticoagulant-antiplatelet therapy often pose the challenging question whether to suspend, reduce, or not to change the treatment because they tend to have more severe epistaxis and bleed from several sites. All patients should have a CBC (complete blood count) and an INR (International normalized ratio) evaluated at the time of presentation. During warfarin-related epistaxis, about 80 % of patients were outside their disease-specific INR range [1].
A multidisciplinary team to optimize safe therapy is often required. A protocol to manage the anticoagulation-antiplatelet related epistaxis is showed below [7]:
Management protocol for anticoagulation-related epistaxis | |
|---|---|
All anticoagulated epistaxis | Order labs: CBC and INR |
Warfarin-related epistaxis | INR in therapeutic range: continue Warfarin INR outside therapeutic range: hold and consult with cardiology or hematology |
Aspirin- or clopidogrel-related epistaxis | Continue medications, but for life-threatening hemorrhage consult cardiology for role of platelet transfusion |
If the hemorrhage is not well managed with the classic anterior-posterior nasal packing, it is often necessary to perform an endoscopic look under general anesthesia or an angiographic study.
6.3 Surgery for Intractable Epistaxis
In the clinical evaluation of epistaxis, posterior bleeding, hematocrit less than 38 % and necessity of blood transfusions seem to be significant predictors of the need for surgical treatment [8].
It has been demonstrated that an early surgical intervention compared to prolonged posterior nasal packing results in a significantly shorter hospital stay and reduced health care costs [1, 9].
Since the mid-1960s, surgical control of posterior epistaxis involved a Caldwell-Luc approach with transantral ligation of the IMA [10]. This external intervention was associated with high patient morbidity and often failed to control the epistaxis because of collateral circulation supplying the SPA distal to the IMA ligation site.
Over the years there has been a progressive refinement of techniques and an increasingly shift toward the distal point of ligation of the vessel. So the external carotid artery ligation (ECAL) has been progressively replaced by the ligation of the IMA, while in the 1970s, the first endoscopic occlusion of the SPA was described [4].
The following text will mainly focus on two endoscopic surgical approaches for intractable epistaxis, both performed under general anesthesia, comparing them to the selective arterial embolization:
- 1.
TESPAL – Transnasal endoscopic sphenopalatine artery ligation
- 2.
TEAEAL – Transnasal endoscopic anterior ethmoid artery ligation
But it is important not to forget a very rare but life-threatening cause of epistaxis: internal carotid artery (ICA) injury during endonasal approaches to the sphenoid sinus.
6.3.1 Transnasal Endoscopic Sphenopalatine Artery Ligation
TESPAL begins with thorough nasal preparation, such as nasal packing removal, nasal cavity cleaning of all blood clots, and decongesting through pledgets soaked with 1 % xylocaine, in order to perform a detailed endoscopic evaluation with a 4 mm 0° optics and define the site of bleeding.
After medializing the middle turbinate, it follows lateral nasal wall incision posterior to the maxillary sinus and a subperiosteal elevation of the mucosa. The ethmoid crest is so identified, then SPA tented up at the level of the SPF and subsequently cauterized (bipolar or Dessi clamp) or clipped. The elevated nasal mucosa is then redraped. In some cases, patients do not need nasal packing and can be discharged in the first postoperative day. Some authors indicate that SPA cauterization, rather than clipping, provides a higher success rate [11, 12].
6.3.2 Transnasal Endoscopic Anterior Ethmoid Artery Ligation
AEA-related epistaxis is less common than the SPA. Its ligation was traditionally performed with an external Lynch incision and clipping between the lamina papyracea and periorbita.
As already described above, a preoperative CT scan of the sinuses is essential to review the AEA anatomy to determine whether it is amenable to endoscopic ligation.
TEAEAL starts with a maxillary antrostomy and anterior ethmoidectomy, then the ethmoid roof and the lamina papyracea are defined. A small opening in the lamina papyracea using a small curette is made below the AEA canal after its identification (image-guidance navigation may be helpful).
After unshelling the bony fragments off the AEA adjacent to the lamina papyracea and their posterior and anterior elevation, small clips are placed across the AEA next to the orbital periosteum [13].
Before choosing to perform TEAEAL, it is important for the surgeon to weigh the potential benefits of avoiding an external scar from the traditional external approach, with the potential for serious complication complications such as cerebral spinal fluid leak, orbital injury, or failure to control epistaxis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







