CHAPTER 8

PREOPERATIVE PREPARATION OF THE EMERGENCY GENERAL SURGERY PATIENT
Skillful perioperative care of acutely ill patients is a defining characteristic of the specialty of acute care surgery. Appropriate resuscitation and planning permit a safe operation and establish the patient’s clinical trajectory. Prevailing literature on preoperative assessment emphasizes risk modification primarily for the elective patient. In an emergency situation, this luxury disappears, and the surgeon must attempt to reverse existing damage and prevent further loss quickly and based on limited information. The acute care surgeon encounters a wide spectrum of patients, with varying severity of chronic and acute illness. Although each patient will provide a unique challenge, the same concepts can be applied universally. It is helpful to follow an approach to the emergency general surgery patient similar to that to a trauma patient—initial evaluation and treatment based on urgency, followed by careful system evaluation prior to surgery.
PRIMARY SURVEY
Identification and Assessment
There is little doubt that the floridly ill 80-year-old woman with free air requires more attention prior to surgery than the healthy 18-year-old male with classic signs of early appendicitis. In between the two extremes are patients who may appear well on preliminary exam only to decompensate in the operating room later. Early identification of patients with subtle signs of shock can greatly affect their outcome when appropriately treated prior to surgery. Clinical assessment of these patients can often be limited, given the time constraints in some emergency situations.
The approach to the emergency surgery patient should include an initial brief history and physical to guide further decision making. Once a surgical emergency is established, the initial medical history can follow that of the “AMPLE” format used for obtaining a history in trauma patients. The “A” and “M” represent a llergies and m edications, for which particular attention should be paid to prescribed anticoagulants, insulin, and cardiovascular medications. The “P” prompts for listing of p revious surgery or prominent medical problems. The “L” indicates the l ast meal and signifies a higher aspiration risk. The “E” refers to e vents that surrounded the inciting event. In the case of the emergency general surgery patient, this should focus on components of the acute illness that may attest to its severity.
Physical examination should have two components: one that focuses on the specific disease process and one that focuses on signs of systemic sequelae. The first identifies the need for surgery; the second quantifies the degree of illness. Establishing the diagnosis by physical exam is fundamental to general surgery practice. Examining the patient for systemic illness is of equal importance to the acute care surgeon. Criteria for systemic inflammatory response syndrome (SIRS) are nonspecific1 but highly sensitive for any type of systemic duress experienced by the patient (i.e., bleeding or tachyarrhythmia) and should prompt further investigation (Table 8.1). Modifications to SIRS scoring can be used to increase specificity for particular systemic illness. To facilitate early identification and treatment of surgical sepsis, for example, we devised a validated screening tool that combined SIRS criteria with a second-level screen to extrapolate possible infectious processes.1,2 Signs of shock can be obvious with tachycardia and hypotension, but cellular hypoperfusion may be ongoing without such outward signs. Signs of pending organ dysfunction and failure should be sought (Table 8.2).
TABLE 8.1
SYSTEMIC INFLAMMATORY RESPONSE SYNDROME (SIRS) CRITERIA

NOTE: Two out of four criteria establish the diagnosis for SIRS.
TABLE 8.2
SIGNS AND SYMPTOMS OF ORGAN DYSFUNCTION
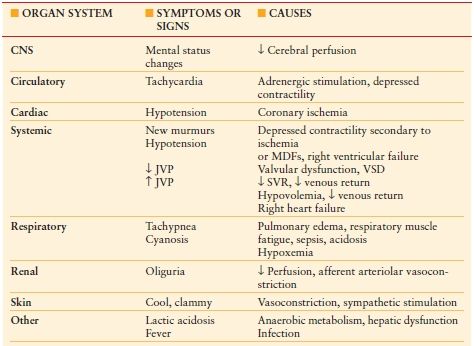
CNS, central nervous system; MDFs, myocardial depressant factors; VSD, ventricular septal defect; SVR, systemic vascular resistance
Basic laboratory studies and x-rays can be valuable adjuncts to identifying the patient with systemic illness. Values on the general chemistry panel, such as low serum bicarbonate and elevated anion gap, can indicate tissue hypoxia. A concentrated hematocrit may also attest to hypovolemia. More specific indicators such as a base deficit, elevated lactate, or decreased oxygen saturation on a venous blood gas can confirm shock and guide resuscitation. A preoperative chest x-ray in the patient with cardiac or pulmonary disease can guide perioperative modalities.
Resuscitation
Similar to the trauma evaluation, the first encounter the surgeon has with the patient in need of emergency surgery may require immediate life-saving measures. Often the patient with presumed surgical pathology will be triaged as such by the emergency department and given less-than-astute attention once the surgeon has been contacted. The surgeon may then arrive to find the patient metabolically deranged to the point of extremis. Simple basic life support skills are then necessary.
Airway and Breathing This is not such an anatomical issue as it is with trauma patients, but the patency of the airway should still be evaluated. These patients may suffer from altered mentation secondary to shock and require airway protection. Tachypnea is often present to compensate for metabolic acidosis and is not well tolerated in the older patient. The use of accessory muscles of breathing, paradoxical respiratory effort, and apnea should all be signs to intubate and ventilate. Pulse oximetry can be used to assess adequacy of oxygenation; however, this can lag behind actual alveolar oxygenation, and a low-normal value should always be interpreted with caution. Anecdotally speaking, if it occurs to you that maybe you should intubate the patient, then you should.
The key to successful intubation is preparation. Always ensure that the appropriate personnel and equipment are available while providing oxygen by mask. Rapid sequence intubation may be achieved with an inducing agent and short-acting paralytic. Etomidate is often favored due to rapidity of action and relative lack of hemodynamic effect. The transient adrenal suppression induced by etomidate has raised concern, and studies are mixed regarding its safety for single use.3,4 Chest x-ray should always be obtained to visualize the tip of the endotracheal tube and avoid right mainstem bronchus intubation for the duration of the ensuing operation.
Circulation Again, trauma resuscitation basics should be followed but are often neglected. Intravenous access upon evaluation of these patients is typically poor—a single 22-gauge peripheral in the hand is simply not adequate. If the classic “two large-bore peripheral IVs” cannot be obtained, then central venous access is necessary, preferably of large diameter, and placed in the chest or neck with ultrasound guidance.
Despite the type of shock, volume loading is the first step of resuscitation. Options include isotonic crystalloids, hypertonic saline, colloids, and blood products. Crystalloids are inexpensive and readily available. Initial bolus of 20 mL per kg of either normal saline or lactated Ringer’s solution should be followed by reassessment and ongoing hydration. Hypertonic saline is promising for emergency use in that it may cause less edema and has a favorable immunologic profile. This has been particularly studied in patients with traumatic brain injury.5 While relatively safe compared to colloid infusion, the administration of large amounts of saline for volume resuscitation carries the concern for hypernatremia, hyperosmolarity, and hyperchloremic metabolic acidosis.
Colloids may provide a greater volume expansion per amount infused, although the presence of leaky capillaries may negate this advantage. Given that it offers no advantage to crystalloid use and is of limited availability, albumin should be limited to specific patient populations.6 Hydroxyethyl starch (HES) has likewise been proposed as a resuscitative colloid. Large volumes of HES should be used with caution, as doses exceeding 20 mL/kg/d can incite platelet dysfunction and renal failure in certain patient populations.7,8
Provision of blood products as a resuscitation fluid should be considered carefully. The risk of infection, immunosuppression, and transfusion reaction are well known.9 Fresh frozen plasma (FfP), cryoprecipitate, and platelets also have utility as colloids based on the coagulation profile. Each has the same adverse transfusion profile as administering packed red blood cells (pRBCs) and should only be used in combination for hemorrhagic shock or for coagulopathy. The patient with ongoing bleeding should be treated with a hemostatic resuscitation strategy that involves transfusion of plasma, platelets, and pRBCs in a ratio that mimics that of whole blood and avoids large volumes of crystalloid infusion.10 Monitoring ongoing transfusion with platelet count, hematocrit, thromboelastrography (TEG), and venous oxygen saturation will help guide further therapy. Hypotensive resuscitation in the hemorrhagic patient is an additional emerging concept.11 Measures to raise blood pressure (BP) with fluid administration may be counterproductive until bleeding is controlled. This approach should be used with caution as efforts to maintain vital organ perfusion (particularly the brain) should be the top priority.
Ongoing signs of shock despite adequate volume resuscitation will require the addition of vasopressors or inotropes. Sympathomimetics are the standard for raising the mean arterial pressure (MAP). Each vasopressor has advantages and disadvantages, although norepinephrine has emerged as one of the first-line agents for septic and cardiogenic shock.12 Inotropes such as dobutamine or milrinone can be added if cardiac output remains low despite adequate volume loading and vasopressor provision. More advanced monitoring should be applied to help titrate inotrope effect.
Adjunctive measures to assist with hemorrhagic or septic source control may be considered as part of the resuscitation strategy. If interventional radiology is available and capable, a well placed drain or coil can stabilize the patient who is otherwise too acutely ill for the operating room. Shock resuscitation should be ongoing throughout this process with the intent of proceeding to the operating room for definitive control when it is safe to do so.
Endpoints of Resuscitation Deciding where surgical intervention fits into shock resuscitation may be difficult. For the less ill patient, providing a liter of crystalloid may be sufficient to restore signs of tissue perfusion prior to operating. For sicker patients, the surgical intervention is an integral part of the resuscitation and must be undertaken before any further improvement can be seen. More difficult yet, particularly in the elderly population, is the nonresponder for whom a surgical intervention will have no bearing on outcome and may put simply put the patient and family through undue duress. Luckily, most patients will fall short of this extreme.
The surgeon should have in mind what end points are to be met before operating. It is prudent that once resuscitation is initiated, the progress of the patient is closely monitored. All too often, an initial assessment and plan will be established, only to find several hours later that nothing has been accomplished. Likewise, “resuscitation” does not equal “extra few hours of sleep” for the surgeon whose patient presents in the middle of the night. While consideration should be given to availability of appropriate operating room staff based on time of day, physiologic optimization of the patient should take precedence.
For the patient in shock, various hemodynamic end points and modes of monitoring progress can be used both pre- and postoperatively. Basic monitoring in patients with shock includes heart rate and urinary output. Noninvasive BP measurements tend to give falsely elevated readings in patients who are hypotensive. An arterial catheter should therefore be placed when there is a concern for hypoperfusion. Central venous pressure (CVP) is often one of the first invasive measures employed. Although this has numerous confounders, resuscitation protocols often aim for a CVP of 12–15 mm Hg.13 With the addition of pulmonary artery catheters, numerous additional hemodynamic parameters become available, although it is not clear that the appropriate end point is the normalization of these values, nor is it clear how these end points should be achieved. Arterial pressure waveform analysis is a promising modality. Utilizing an existing arterial catheter, it can provide reliable measures of cardiac output and volume status that can outperform pulmonary artery and central venous catheters for resuscitation.14
End points of tissue oxygenation may also be used for resuscitation. Mixed-venous oxygen saturation (SvO2) has classically been employed as an estimate of global tissue perfusion, but requires a pulmonary artery catheter for measurement. Values <65% are associated with reduction in oxygen delivery, indicating reduced cardiac output or oxygen content of blood. Central venous oxygen saturation (ScvO2) generates similar information from a standard central venous catheter, with values <70% significant.15 Laboratory values such as lactate and base deficit can also help guide resuscitative efforts, with prognostic significance.16 Near-infrared spectroscopy offers a method of monitoring tissue hemoglobin oxygen saturation (StO2) in the skeletal muscle. Changes in skeletal muscle StO2 correlate well with changes in oxygen delivery, base deficit, and lactate levels during active resuscitation.18
SECONDARY SURVEY: SYSTEM-BASED CONSIDERATIONS
A surgical emergency can often be the patient’s first exposure to the health care system, leaving the surgeon to uncover previously undiagnosed medical problems. Special attention in the history should be given to previous problems with anesthesia, clotting disorders, as well as medications and substance use. Baseline functioning including mental status, activity level, and vital signs should also be established from family if the patient is unable to communicate. Careful physical examination can reveal other pertinent comorbidities. These include signs of pulmonary disease, vascular disease, cirrhosis, previous unmentioned surgery, and malnutrition, to name a few. The following describes components of the preoperative evaluation by system, with more pertinent topics discussed in detail.
Neurologic
Baseline function should be established through either the patient or family, particularly for elderly patients who may have dementia. Superimposed delirium from acute illness complicates this exam. Chronic neurologic disease should be ascertained, as numerous diseases such as muscular dystrophy, myasthenia gravis, multiple sclerosis, epilepsy, Parkinson’s disease, and Alzheimer’s disease may influence the type of anesthetic and paralytic agents used.
Substance Abuse and Dependency Patients undergoing emergency surgery often present with substance use disorders. Alcohol dependency poses the most dangerous risk to the patient in the perioperative period. Acute illness may impact the usual amount of alcohol ingested, initiating withdrawal sooner than expected. Administration of benzodiazepines prior to surgery reduces the dose of inhaled anesthetics required and prevents seizures. Preoperative morphine can help mediate the exaggerated stress response that is chronically suppressed by alcohol.19 For the malnourished alcoholic, thiamine should be given prior to glucose administration to prevent Wernicke’s encephalopathy. Nicotine and narcotic dependence should also be anticipated and treatment begun in the preoperative period. When initiated early, nicotine replacement therapy can reduce opioid requirement.20 Narcotic dependence can be treated with short- as well as long-acting opioids, with clonidine added to help temper withdrawal symptoms.21
Analgesia Pain control prior to surgical evaluation is an area of debate. Concern for “masking the examination” hinders administration of appropriate analgesia and unnecessarily leaves the patient in pain. Meta-analysis of adult and pediatric patients treated with opiates shows a trend toward increased risk of altered physical exam, but no difference in subsequent management thereof.22 Patients with chronic pain syndromes should be treated appropriately with their baseline dosing plus additional narcotics as needed for acute pain.
Chronic Pulmonary Disease Patients with preexisting chronic pulmonary disease present an additional challenge for the surgeon. Emergency patients do not have the luxury of undergoing optimization or risk stratification before surgery, and therefore may present with ongoing atelectasis, respiratory infection, or bronchospasm. Smoking history and chronic obstructive pulmonary disease are clear risk factors for postoperative pulmonary complications such as pneumonia, pleural effusion, and respiratory failure.23 Additional preoperative risks are low oxygen saturation, respiratory infection within the past month, age >80 years, and preoperative hemoglobin <10 mg per dL.24 Avoiding these complications can be difficult in the immediate preoperative period, but a few strategies for risk reduction can be applied (Table 8.3).
TABLE 8.3
PREOPERATIVE INTERVENTIONS TO REDUCE THE RISK OF POSTOPERATIVE PULMONARY COMPLICATIONS
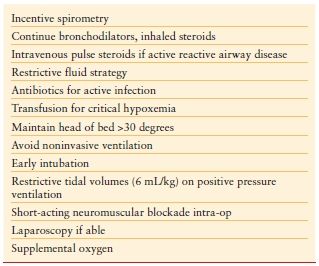
Acute Lung Injury Acute lung injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) are particularly devastating for the emergency patient. The combination of aggressive resuscitation strategies, profound inflammatory processes, and blood transfusions adds to this risk. Strategies to limit further barotrauma with restrictive tidal volumes (6 mL/kg), judicious use of positive end-expiratory pressure, and permissive hypercapnia have been described once the patient is diagnosed with ALI/ARDS.25 Studies have also demonstrated that application of a low tidal volume protocol from the outset can prevent subsequent ALI/ARDS by limiting pulmonary cytokine production and inflammation.26,27 While the initial tendency will be to increase s ventilation in these patients to help compensate for metabolic acidosis, these measures may lead to subsequent barotrauma, and use of lower tidal volumes should be encouraged.
Cardiovascular
Guidelines for preoperative cardiac assessment are abundant and revised frequently. These algorithms uniformly divert the patient directly to surgery for true emergency situations. Patients with acute coronary syndrome complicating their surgical disease should be treated according to American Heart Association/American College of Cardiology (AHA/ACC) guidelines for coronary revascularization.28 Any attempt to temporize the acute surgical problem should be made in the face of ongoing cardiac ischemia. Coordinating care in a multidisciplinary manner for these patients is paramount. For the more common patient with stable cardiac disease, preparation for surgery is much less dramatic. The limited workup for these patients derived from the history, physical exam, and EKG can provide information regarding functional status. Preoperative optimization options are limited in a short time frame, but a few basic principles can be applied (Table 8.4).
TABLE 8.4
PREOPERATIVE INTERVENTIONS TO REDUCE THE RISK OF POSTOPERATIVE CARDIAC COMPLICATIONS
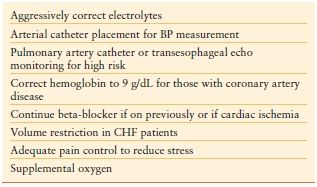
Hypertension Although preoperative hypertension is an important predictor of postoperative morbidity, no data have established that this is remedied by preoperative treatment. This applies principally to those with long-standing moderate hypertension for which any attempt at acute reduction may result in relative hypoperfusion once general anesthesia is induced. Stage III hypertension (>180/110 mm Hg), however, requires control prior to surgery. Continuous intravenous esmolol, labetalol, nitroprusside, or nitroglycerin may be used, as may some of the newer calcium channel blockers such as nicardipine or clevidipine.29 Invasive arterial BP monitoring for these patients is essential.
Beta-blockade Beta-blockers mitigate cardiovascular stress from surgery by suppressing catecholamine production and increasing stability of atherosclerotic plaques. Initial data regarding perioperative beta-blocker use in cardiovascular surgery demonstrated a decrease in myocardial ischemia, ventricular arrhythmias, and blunting of the hypertensive response in high-risk patients.30,31 This led to a class I recommendation by the AHA for perioperative beta-blockade with a goal heart rate of 60 bpm for those patients with coronary ischemia undergoing vascular surgery. A class IIa recommendation was also made for patients with untreated hypertension, coronary artery disease, or other risk factors.32
With this evidence, perioperative beta-blockade was enthusiastically and liberally applied. Emerging data began to demonstrate unacceptable risks of bradycardia, hypotension, and stroke in certain patient populations.33–36 When stratified per cardiac risk index, the relationship between perioperative beta-blockade and risk of death varied directly with cardiac risk: those with high risk (cerebrovascular or coronary artery disease, insulin-depending diabetes, creatinine >3 mg/dL,) showed a benefit, but those with low risk showed a trend toward harm.37 Subsequent revisions to the ACC/AHA guidelines restrict class I indication for perioperative beta-blockade to those already on beta-blockers and to patients with known coronary ischemia undergoing high-risk surgery.38
Congestive Heart Failure Patients with congestive heart failure (CHF) should be cautiously managed prior to emergency surgery. Therapy is aimed at reducing ventricular filling pressures in addition to improving cardiac output. This may be a difficult balance in the face of ongoing disease processes such as vasodilatory shock or bleeding. Chronic disease may be complicated by acute ischemia or metabolic myocardial depression. Inotropes such as dobutamine or milrinone may be needed for those with systolic dysfunction. More invasive monitoring devices such as pulmonary artery catheters or continuous transesophageal echocardiography should be available intraoperatively for this patient population.
Preoperative administration of chronic medications used to treat CHF should be considered carefully. Beta-blockade has already been discussed and should be continued for those already on therapy but without ongoing hypotension.39 Diuretics should be administered only if clinically indicated on physical exam. Ongoing angiotensin-converting enzyme (ACE) inhibitor administration should be avoided in those patients with hypotension or acute renal insufficiency, but otherwise may have a protective effect.40 Digoxin should be held in the preoperative period, as therapy with the drug is associated with an increased cardiac risk in urgent surgical patients.41
Arrhythmias Perioperative atrial arrhythmias can contribute substantially to morbidity in the emergency surgical patient. Electrolytes should be aggressively corrected and home medications used for rate or rhythm control continued. Acute-onset atrial fibrillation with rapid ventricular response should be stabilized according to advanced cardiac life support protocol prior to proceeding to the operating room.42 New arrhythmias should prompt investigation for ischemia, for which reperfusion may take precedence of acute surgical pathology. Patients with permanent pacemakers or implantable defibrillators should be identified preoperatively. If time permits, the device should be interrogated by the electrophysiology service. Otherwise, a magnet should be taped over the device en route to surgery. Magnets will reprogram pacing devices to asynchronous mode and will disable defibrillators, making them immune to electromagnetic interference that may be experienced in the operating room.
Renal
Acute kidney injury (AKI) is unfortunately commonplace in the emergency surgery patient and portends a bad outcome. The surgeon should correct issues such as hypovolemia, electrolyte disturbances, and acid/base prior to operating. Metabolic acidosis should be initially treated with volume resuscitation and correction of the underlying problem. If acidosis persists and is hemodynamically limiting, then temporizing measures with sodium bicarbonate, tromethamine (THAM), or immediate continuous renal replacement therapy should be considered.43 Additional indications for emergent dialysis in the preoperative period (volume overload, intractable hyperkalemia, azotemia, pericarditis), whether for acute or chronic renal failure, should be considered and pursued if time permits.44 For the patient with chronic renal failure, the surgeon should also be cognizant of appropriate positioning on the table to avoid compression of existing arteriovenous fistulas, as well as avoiding the subclavian vein for central venous catheter placement.
Acute Renal Dysfunction Most cases of renal impairment share the underlying pathology of hypoperfusion.45 Pre-existing renal function and patient comorbidities such as hypertension, diabetes, and vascular disease play a critical role. Inflammatory and nephrotoxic factors, in combination with type of surgery, can exacerbate these baseline factors. Renal perfusion may be preserved by adequate volume resuscitation and goal-directed therapy.46
Contrast-induced Nephropathy Special consideration is given to the acutely ill patient facing exposure to contrast agents for either diagnosis or therapeutic purposes. Current guidelines for prevention of contrast-induced nephropathy (CIN) emphasize provision of isotonic fluid. Normal saline should be administered at 1 mL/kg/h for 24 hours, beginning 2–12 hours before contrast administration. The lowest dose and osmolality contrast should be used. N-acetylcysteine (Mucomyst) is often employed with a standard dose of 600 mg every 12 hours for four doses beginning before contrast is given. Although this drug is promising as a free-radical scavenger, the data show no improvement in renal function, despite a decrease in creatinine.47 Meta-analysis of sodium bicarbonate for prevention of CIN likewise shows no benefit over isotonic saline administration.48 Other nephrotoxic agents should likewise be avoided. These include ACE inhibitors, angiotensin II receptor blockers, and metformin.
Gastrointestinal
The emergency general surgery patient very often has some component of ileus and is therefore always considered at high aspiration risk. A nasogastric tube should be placed prior to induction of anesthesia to help empty the stomach and prevent voluminous aspiration. Premedication with H2-receptor blockers is typically given in this patient population prior to induction as well. If the need for future enteral feeding access is anticipated, appropriate tubes can be made available in the operating room. Signs and symptoms of liver disease should be carefully elucidated. Further management of the cirrhotic is discussed below.
Abdominal Compartment Syndrome Intra-abdominal hypertension and abdominal compartment syndrome (ACS) should be considered in certain patients presenting with emergency abdominal pathology. The triad of oliguria, elevated peak airway pressures, and elevated intra-abdominal pressure (as obtained from bladder pressure readings) defines ACS, which can be from either a primary or secondary process.49 When suspected or diagnosed preoperatively, the surgeon should proceed expeditiously to the operating room to avoid bowel ischemia and further kidney injury. Plans for leaving the fascia open should be made prior to starting the operation so that appropriate supplies are available.
Hematology
Transfusion The concept of liberally transfusing the patient prior to proceeding to the OR with the intention of “loading them up” should be abandoned. While certain patient populations are critically dependent on optimization of oxygen content of blood, most patients needlessly risk the adverse effects associated with transfusion. Patients with active bleeding are the exception, and strategies for transfusion in this population have been described above.
Tolerance for a lower hematocrit in critically ill patients has been supported by the Transfusion Requirements in Critical Care (TRICC) trial. This demonstrated that a restrictive transfusion strategy (hemoglobin trigger of 7 g/dL) is as at least as effective and may be superior to a liberal transfusion threshold of 10 g per dL.50 This finding persisted for patients with coronary disease, with the exception of those with an acute ischemic event or unstable angina.51 This was a select group of ICU patients who were considered to be euvolemic, and thus patients with active hemorrhage were excluded in this study and were not specifically identified.
A recent study using the National Surgical Quality Improvement Program (NSQIP) database examined patients undergoing noncardiac surgery.52 An increase in mortality was shown for those older than 65 years when preoperative hematocrit levels deviated from normal. This was an observational study, however, and patients undergoing emergency surgery did not experience the same effect. A recent large prospective study likewise examined a restrictive versus liberal (hematocrit 24% vs. 30%) transfusion policy prior to cardiac surgery.53 Thirty-day all-cause morbidity and mortality were the same for both groups; however, the number of units transfused was an independent risk factor for subsequent complications.
Anticoagulation Reversal When performing emergency surgery on anticoagulated patients, the surgeon must balance the risk of operative bleeding versus thromboembolism. For the patient with minimal clinical consequence of operative site bleeding and high risk of thromboembolism, a higher threshold should be given to acceptable INR levels or continuing anticoagulant therapy. High-risk patients with prosthetic valves in the mitral position, atrial fibrillation associated with mitral valve disease, and a history of thromboembolism should be placed on intravenous heparin therapy while warfarin is held. In this patient population, FTP may be administered to acutely reverse anticoagulation, with care taken to avoid volume overload. Vitamin K therapy should be avoided as the delayed effect will serve no purpose in the short term and complicate subsequent anticoagulation in the postoperative period.
Similarly, patients taking antiplatelet therapy should be treated with a risk/benefit approach. Prevalence of patients taking dual antiplatelet therapy with aspirin plus clopidogrel is increasing with expanding criteria for use in cardiovascular patients. Patients with bare metal coronary stents placed within the last 6 weeks or drug-eluting stents placed within the last 12 months should be considered at high risk of a fatal perioperative myocardial infarction.54 As such, if bleeding risk is acceptable, the patient should be left on antiplatelet therapy without attempt to reverse the effects of platelet inhibition. For the patient on antiplatelet therapy without high risk of cardiac or cerebrovascular complications, a strategy to temporarily reverse platelet inhibition is desired. Ideally, platelet reactivity could be assessed by light transmittance aggregometry, but this test may be of limited availability, particularly in an emergency setting. If the bleeding risk is high, two to three pools of platelet concentrate should be administered to temporarily assist with clot formation. This strategy has been demonstrated to normalize platelet response in those treated with typical doses of clopidogrel plus aspirin.55
Thromboembolism Prophylaxis The risk for venous stasis and subsequent thromboembolic disease increases in the operative period due to vasodilation, inflammation, and immobilization. Most hospitals include the placement of mechanical antithrombotic devices (e.g., sequential compression devices) in the operating room checklist and emphasize placement prior to induction of anesthesia. Pharmacologic methods for deep venous thrombosis (DVT) prophylaxis are often not emphasized preoperatively; however, trials demonstrating heparin efficacy were performed with dosing started 1–2 hours before surgery.56 Enoxaparin and subcutaneous heparin are therapeutically equivalent for preventing DVT, although renal clearance of enoxaparin makes dosing less predictable in those patients with compromised kidney function.57 Patients at high risk of bleeding should at least have mechanical methods employed in the operating room.58
Endocrine
Hyperglycemia Observational evidence shows that patients with acutely elevated glucose preoperatively fare worse than those who are normoglycemic; however, no trials investigate whether acute correction demonstrates any benefit.59 In 2001, Van den Berghe et al. published a large randomized controlled trial of surgical intensive care unit patients in which intensive insulin therapy (IIT) (target blood glucose [BG], 80–110 mg/dL) reduced in-hospital mortality by 34% when compared to standard therapy (target BG, 180–200 mg/dL).60 This finding has been broadly applied and included in perioperative care guidelines, as well as included as a Surgical Care Improvement Project (SCIP) measure. Particular attention has been given to achieving normoglycemia intraoperatively, although results are mixed. Higher incidence of clinically manifest hypoglycemia, in addition to the findings from the NICE-SUGAR trial, has generally called into question the safety of IIT.61 Guidelines from major societies now advocate a “reasonable, achievable, and safe” approach to tight glucose control acutely.62
Diabetic Ketoacidosis Diabetic ketoacidosis (DKA) is a serious diabetic emergency and should be aggressively treated preoperatively. The American Diabetic Association (ADA) defines DKA as glucose > 250 mg per dL, pH < 7.3, serum bicarbonate < 18 mmol per L, anion gap > 10, and presence of ketosis.63 Typically, an infectious process initiates the process, so the emergency surgery patient with diabetes is at high risk. Treatment consists of aggressive hydration and provision of intravenous insulin. Electrolytes should be monitored every 2 hours, and hypokalemia corrected. When the serum glucose falls below 250 mg per dL, the IV fluid should be changed to 5% dextrose with 0.45% sodium chloride with subsequent glucose goal of 150–200 mg per dL. In the emergency setting, this process should be pursued at least with the goal of providing generous hydration and correction of hypokalemia prior to proceeding to the operating room.
Stress Dose Steroids Patients treated in the long term with exogenous steroids have a blunted hypothalamic–pituitary–adrenal axis response to surgical stress when compared to normal controls. Complete cessation of steroids in the perioperative period for those on long-term treatment can result in a 1%-2% incidence of hypotensive crisis with significant risk of death.64 Based on these findings, the concept of “stress dose steroids” has been widely applied and is now dogma. There is no evidence that supraphysiologic doses of corticosteroids are necessary to prevent hemodynamic instability however. Numerous studies have failed to demonstrate any difference in perioperative BP when comparing normal steroid dosing with supraphysiologic levels.65,66 Given the risks of corticosteroids (wound healing, immunosuppression, interaction with anesthetic agents, psychiatric disturbances), the concept of “stress dosing” above the baseline should be reexamined. Current guidelines recommend providing the patient with an intravenous dose equivalent to their baseline until oral medications can be resumed.67 An important exception to this recommendation is the critically ill patient. Steroid-dependent patients requiring vasopressors should be tested for adrenal insufficiency and started on supraphysiologic doses of 100–150 mg intravenous hydrocortisone daily until hypotension resolves.68
Infectious Disease
Sepsis A large percentage of patients undergoing emergency surgery by the general surgeon will have an infectious etiology. For those patients without systemic signs of disease, management is straightforward: source control (surgery) and/or antibiotics. For those patients with sepsis (SIRS plus infection), management requires more skillful resuscitation. Treatment is based on primary source control, with resuscitative efforts to limit systemic inflammatory sequelae. The specifics of sepsis management are discussed later in the chapter as it pertains to intra-abdominal catastrophe.
Surgical Prophylaxis Surgical prophylaxis refers to the administration of antibiotics in the patient with no signs of infection in an effort to reduce the risk of postoperative wound infections. Clean cases in which the gastrointestinal tract, genitourinary tract, or respiratory tract are not entered do not routinely require antibiotic prophylaxis. This is rarely the case for emergency general surgery. Antibiotics should be chosen based on operative site and predominant bacterial flora to that area. Skin flora such as staphylococci and streptococci should be covered for most cases, with additional coverage of gram-negative flora and anaerobes for cases involving the gastrointestinal tract. Antibiotics should be redosed intraoperatively based on clearance profiles for that drug, as well as for cases in which blood loss is estimated over 1.5 L.
Guidelines from the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) and SCIP provide common recommendations to further limit surgical site infection.69 Antibiotics should be administered within 1 hour before surgical incision, but should be completed prior to skin incision to achieve maximal tissue penetration. True antibiotic prophylaxis should be completed within 24 hours of the case. Hair at the operative site should be clipped (not shaved). Additionally, hypothermia and hyperglycemia should be avoided. Specific antibiotics for use in general surgery are outlined in Table 8.5.
TABLE 8.5
SURGICAL ANTIMICROBIAL PROPHYLAXIS. COVERAGE FOR GENERAL SURGERY CASES—GASTROINTESTINAL
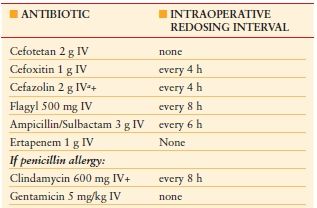
a Substitute vancomycin for cefazolin in hospital with high MRSA rate
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








