SUPPORT OF THE ORGAN DONOR
The gap between the availability of organs and the demand for transplantation is large. The number of potential organ donors in the United States has been estimated to be more than 10,500 per year,1 and, clearly, not all potential donors progress to organ procurement. As of July 22, 2006, the United Network for Organ Sharing (UNOS) stated that the number of patients awaiting transplantation in the United States was 92,450. Looking at data for the period 1997 through 1999, only 50% of health care agents who were proxies approached regarding donation agreed to proceed,1 yielding a donor conversion rate of 42%. Factors affecting procurement include the number of families approached regarding organ donation, families’ perception of the donor process, religious or spiritual beliefs, cosmetic appearances, and the difficulty regarding change in provider focus from the health of their patient to the health of an unseen patient at a different location. Steps being taken to decrease the gap between demand and availability include the use of living donors, expanded criteria donors (ECDs), and donation after cardiac death (DCD, or non–heart-beating donor). As critical care surgeons, it is vitally important to understand this demand as well as our potential role in maximizing opportunities for organ procurement. At the same time, we must remain focused on the care of our patients and their families, as well as being respectful of their wishes.
In this chapter we explore the roles that the acute care surgeon plays in support of the potential organ donor. We will also examine issues surrounding the declaration of brain death, non–heart-beating donors, and medical–legal implications surrounding organ donation.
ROLES OF THE ACUTE CARE SURGEON IN CARING FOR POTENTIAL ORGAN DONORS
Determination of Death
Due to the nature of acute care surgery, surgeons practicing in this field will often be required to assist in the determination of death. This is often difficult as the focus now changes from preservation of the patient’s life to declaration of death and possible salvage of a patient unknown to the surgeon. With regard to patient death and donation, one source of uncertainty that is important to manage is the application of brain death criteria or criteria for DCD.
One of the important guiding principles of organ transplantation is the “dead donor rule.” This rule, a general ethical principle in organ transplantation, declares that organ procurement should occur only when the patient is dead. The Uniform Determination of Death Act (UDDA) defines death based on either the irreversible cessation of: (1) circulatory and respiratory functions, or (2) all brain functions, including the brain stem.
In 1968, an Ad Hoc Committee of Harvard Medical School defined brain death as “the irreversible cessation of all brain functions,” thereby permitting transplant surgeons to harvest organs from a donor whose heart and lungs were still functioning.2 The following are the guidelines for the determination of brain death in adults:
- The patient must be in a deep unresponsive coma, there must be no movement or responses to stimulation, and there must be a clear understanding as to the etiology of the coma.
- The patient must have a core temperature that is at least 32°C (90°F)
- Drug intoxication and poisoning must be excluded.
- There are no medical conditions that may confound the clinical assessment of brain death (e.g., no severe electrolyte, acid-base, or endocrine disturbances)
- The patient must be apneic and must not recover spontaneous respiratory function during an apnea test.
Apnea Test:
- Core temperature 36.5°C (97°F)
- Systolic blood pressure 90 mm Hg
- Corrected diabetes insipidus or positive fluid balance in past 6 hours
- Patient on 100% oxygen at appropriate intermittent mandatory ventilation (IMV) for pCO2 36–45 mm Hg, pH 7.35–7.44 for 30 minutes; blood gas just prior to apnea test to confirm pCO2 36–45 mm Hg
- Place on continuous positive airway pressure (CPAP) at FIO2 100% or place on T-piece 100% FIO2 at >6 L/min
- Note the presence or absence of spontaneous respiration during or at the conclusion of 10-minute period of observation
- Check arterial blood gases (ABG) after the 10 minutes to confirm pCO2 ≥ 60 mm Hg (Note: if the pCO2 is 60 mm Hg or 20 mm Hg above baseline, the apnea test supports the clinical diagnosis of brain death.
- Core temperature 36.5°C (97°F)
- Two physicians must record the declarations of Brain Death in the progress notes and must sign, date, and time both declarations and document that the patient is brain dead. The clinical examinations may be up to 6 hours apart. The second clinical declaration is considered the legal time of death. The two physicians who independently determine brain death are not permitted to participate in the procedures for procuring or transplanting an organ or other body part from the patient.
- Cranial nerve reflexes and responses:
- Pupils midposition (4 mm) to dilated (9 mm), fixed, and unresponsive to light.
- Absence of corneal reflexes.
- No oculocephalic reflex
- No deviation of the eyes to irrigation in each ear with 50 mL of ice water
- No swallowing or yawning
- No gag reflex
- No cough response or bradyarrhythmia to bronchial suctioning
- Pupils midposition (4 mm) to dilated (9 mm), fixed, and unresponsive to light.
Donation after Cardiac Death
In light of the aforementioned shortage of available organs for transplantation, DCD has emerged. Determining irreversibility depends on the circumstances; irreversible cessation of circulatory and respiratory functions may mean that the heart cannot be restarted spontaneously or that the heart cannot be restarted because of the decision to forego resuscitative measures.2 A “do-not-resuscitate” (DNR) order prevents the physician from engaging in resuscitation so that the patient’s state of death is legally irreversible.
DCD practices pose additional legal considerations because the timing for declaring irreversible cessation of circulatory and respiratory function varies across transplant programs. Therefore, DCD protocols should clearly indicate which time period the hospitals and organ procurement organizations (OPOs) will follow, such as waiting between 2 and 5 minutes after the cessation of cardiopulmonary function before organ procurement.2
UNOS, which operates the Organ Procurement and Transplantation Network (OPTN), has developed rules regarding DCD, which were finalized in March 2007 and became effective on July 1, 2007, requiring that all OPOs and transplant centers must incorporate “model elements” in their DCD protocols.2 These model elements address the withdrawal of life-sustaining measures and pronouncement of death as described below3:
Withdrawal of Life-sustaining Measures/ Patient Management
- A time-out is recommended prior to the initiation of the withdrawal of life-sustaining measures. The intent of the time-out is to verify patient identification and the respective roles and responsibilities of the patient care team, OPO staff, and organ recovery team personnel.
- No member of the transplant team shall be present for the withdrawal of life-sustaining measures.
- No member of the organ recovery team or OPO staff may participate in the guidance or administration of palliative care, or the declaration of death.
- There must be a determination of the location and process for withdrawal of life-sustaining measures (e.g., endotracheal tube removal, termination of blood pressure support medications) as a component of the patient management.
In pronouncing death for DCD, the physician who is authorized to declare death must not be a member of the OPO or organ recovery team, and the method of declaring cardiac death must comply in all respects with the legal definition of death by an irreversible cessation of circulatory and respiratory functions before the actual pronouncement of death.
PATHOPHYSIOLOGY OF BRAIN DEATH
Donor management begins with the neurologic determination of death (NDD, or “brain death”) and consent to organ donation, and culminates in surgical organ procurement. Understanding the pathophysiology of brain death provides an important opportunity to enhance multiorgan function during this period of time and thereby improve organ utilization. It is important to realize that the resuscitation of the cardiopulmonary system benefits function of all organs and it is important to take time in the intensive care unit (ICU) to optimize organ function to improve transplant outcomes. Hence, reversible organ dysfunction can be addressed with aggressive resuscitation; once organ function is optimized, surgical procurement should be arranged emergently. The temporal changes in organ function after NDD demand flexibility in identifying the optimal time for procurement.4
The earliest manifestation of increased intracranial pressure (ICP) is characterized by hypertension, bradycardia, and altered breathing. When the brainstem becomes compressed as it is pushed against the foramen magnum, ischemia will result. This results in increased local neuronal activity that produces an increase in sympathetic outflow and an elevation in blood pressure in order to maintain cerebral blood flow. The hypertension will additionally produce reflex bradycardia. Herniation, however, is associated with a massive increase in catecholamines that produces hypertension and tachycardia. The catecholamine surge, which can lead to coronary vasoconstriction, subendocardial ischemia, and focal myocardial necrosis, is believed to be one of the contributing factors to cardiac dysfunction in potential organ donors. Additionally, the catecholamine surge may lead to increased filling pressures, increased afterload, and an elevated pulmonary wedge pressure (PWP), which then leads to “neurogenic pulmonary edema.” The sympathetic storm lasts for only a brief period of time and is then followed by a prolonged phase of severe hypotension secondary to decreased sympathetic outflow. The hypotension may be further compounded by ventricular dysfunction requiring use of inotropes and vasopressors to maintain organ blood flow. The deterioration of cardiovascular function associated with intracranial hypertension varies with the rapidity of increase in ICP, time after herniation, and etiology of brain injury.5 There is also evidence that brain death induces an inflammatory response and subsequent endothelial injury, which can lead to impaired graft function and graft vasculopathy.
On a cellular level, there is global mitochondrial dysfunction and a change from aerobic to anaerobic metabolism with reduced energy stores in the myocardium. This, combined with the ischemic time and ischemia–reperfusion injury, contributes to graft dysfunction. From an endocrine standpoint, there is a reduction in antidiuretic hormone (ADH, vasopressin) secretion and thyroid dysfunction. The decrease in ADH leads to the development of diabetes insipidus (DI) whereas the thyroid dysfunction generally manifests as hypothyroidism. There is data to suggest that proinflammatory cytokines may inhibit the conversion of thyroxine (T4) to the active form of thyroid hormone, tri-iodothyronine (T3). T3 via binding to specific receptors, increases oxygen utilization, the uptake of glucose and amino acids, mitochondrial size/number, and promotes a conversion to aerobic metabolism. Additionally, T3 will increase cardiac output and decrease systemic vascular resistance.
From a hematologic standpoint, release of thromboplastin from the ischemic brain can cause disseminated intravascular coagulation while the systemic inflammatory response syndrome response activates humoral and cellular components that add to the coagulopathy. As mentioned earlier, a sympathetic storm occurs during herniation which is rapidly followed by a depletion of catecholamines that can lead to significant hypothermia due to loss of central temperature control and vasodilation. Hypothermia can further exacerbate myocardial depression and further compromise oxygen delivery.
OPTIMIZATION OF THE POTENTIAL ORGAN DONOR
Subsequent to the pronouncement of death, the donor management period begins. During the donor management period, the acute care surgeon and organ procurement coordinator should focus on optimizing the patient for donation. Appropriate organ donor management then should address the pathophysiologic changes mentioned previously to optimize the number of organs transplanted per donor (OTPD). The main goals are to maintain cardiac function, minimize the deleterious effects that occur with brain death, and maintain adequate oxygen delivery to the vital organs. These goals can be met by optimizing hemodynamics with the judicious use of fluids, inotropes, and vasopressors, preserving lung function by paying close attention to mechanical ventilation and pulmonary toilet, preventing infection, and by utilizing specific measures shown to maintain and improve cardiac function. Detailed standing orders released by the Canadian Council for Donation and Transplantation are shown in Table 57.1. Note that the placement of invasive monitoring (central venous pressure [CVP], arterial catheter, Foley’s catheter) is typically required.
TABLE 57.1
DATA COLLECTED FROM UNOS REGION 5 DONOR MANAGEMENT GOALS (DMG)



It is important to take the necessary time in the ICU to optimize organ function to improve transplant outcomes. Resuscitation and reevaluation can improve reversible organ dysfunction (myocardial and cardiovascular dysfunction, oxygenation impairment related to potentially reversible lung injury, invasive bacterial infections, hypernatremia) and evaluate temporal trends in hepatic AST, ALT, and creatinine or any other potentially treatable situation. This treatment period can range from 12 to 24 h and should be accompanied by frequent reevaluation to demonstrate improvement in organ function toward defined targets. Once organ function is optimized, surgical procurement procedures should be arranged emergently. There are no demographic factors or organ dysfunction thresholds that preclude giving consent for donation and offering organs for transplantation.
Reprinted with permission from CMAJ. 2006;174(6):S27.
ABGs, arterial blood gases; CVP, central venous pressure; DDAVP, 1-desamino 8-D arginine vasopressin (desmopressin); ECG, electrocardiogram; INR, International Normalized Ratio; LVSWI, left ventricular stroke work index; MVO2, mixed venous oxygen saturation; PAC, pulmonary artery catheter; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; PT, prothrombin time; PTT, activated partial thromboplastin time; PWP, pulmonary wedge pressure; SVR, systemic vascular resistance; SWI, stroke work index; IV, intravenous
The most frequent hemodynamic problem is hypotension due to either hypovolemia, neurogenic shock due to decreased sympathetic outflow, or myocardial dysfunction. Treatment begins with optimizing preload (CVP 6–10 mm Hg) with the most appropriate fluid (crystalloid, colloid, or blood product). If hypotension persists despite adequate filling pressures, an inotrope should be considered. If inotropes are initiated, consideration should be given to the placement of a pulmonary artery catheter (PAC) to assess preload, afterload, and cardiac performance. Simply stated, an escalation of support should be accompanied by escalation in monitoring. Subsequently, if inotrope dosing has to be increased due to persistent hypotension, an investigation into the exact cause must be undertaken. Echocardiography can be used to evaluate cardiac function and if this demonstrates adequate ventricular function and volume status, then severe vasodilation is the most likely cause. In such cases, a vasoconstrictor would be considered as the agent of choice. Vasopressin has been shown to be very useful in this setting because it acts synergistically with catecholamines and may allow a reduction or even discontinuation of them. Vasopressin is a special agent because it can be used in a variety of applications, i.e., hemodynamic vasopressor support, DI therapy, and hormone therapy. Second-line agents for hemodynamic support include norepinephrine, epinephrine, and phenylephrine. Escalation of doses of catecholamines should be guided by PAC data realizing that a reduction in inotropes is desired as it will reduce myocardial oxygen consumption and thereby help maintain myocardial energy stores. Optimization of these parameters, along with attention to acid-base and electrolyte balance, hematocrit, oxygenation and ventilation, and hormone supplementation has been shown to improve potential organ donor salvage.6
Hypoxemia can result in cardiovascular instability and may be due to pneumonia, aspiration pneumonitis, pulmonary contusions, pulmonary edema, ventilator-associated lung injury, and oxygen toxicity. The lowest inspired oxygen concentration, peak inspiratory pressure (PIP) and positive end expiratory pressure (PEEP) compatible with acceptable oxygenation should be used. The quality of donor lungs can be improved with endotracheal suctioning, bronchodilators, steroids, chest physiotherapy (PT), bronchoscopy and lavage, and judicious small-volume colloid resuscitation.
From an endocrine standpoint, central DI occurs in 50%–80% of brain dead patients. Central DI is characterized by decreased secretion of ADH, which gives rise to polyuria and polydipsia by diminishing the patient’s ability to concentrate urine. This results in a loss of intravascular volume and electrolyte abnormalities. Treatment with desmopressin (1-desamino-8-D-arginine vasopressin [DDAVP]) loading followed by an infusion can reduce the volume losses and help correct the electrolyte issues. Due to the multiple endocrine abnormalities, it is now recommended that a hormonal “cocktail” consisting of T3, vasopressin, and methylprednisolone be administered to potential organ donors.7 The weight ofcurrently available evidence in a large retrospective cohort study by the United Network for Organ Sharing in the United States suggests a substantial benefit of triple hormone therapy with minimal risk. This study showed substantial increases in kidney, liver, and heart utilization from donors receiving triple hormonal therapy as well as improvements in 1-year kidney and heart graft survival.8 Glycemic control should be achieved, if necessary by insulin infusion. Additionally, routine enteral feeding should be initiated or continued as tolerated, and discontinued on call to the operating room for harvesting, whereas parenteral nutrition should not be initiated, but may be continued if it had already been started.
IMPLEMENTATION OF THE ORGAN DONOR MANAGEMENT PROTOCOL
Multiple studies suggest that the use of guidelines and protocols to identify and care for the potential organ donor are effective in improving both organ donation rates and the number of OTPD.9–15 These guidelines and protocols must provide clinical management guidance incorporating the principles mentioned previously, as well as the social support necessary to accomplish the task of obtaining consent and supporting the family and friends of the potential donor.
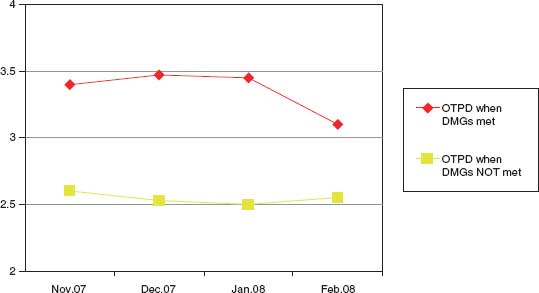
The clinical component of the management protocol should include early identification with admission to a monitored setting and resuscitation in accordance with the principles mentioned earlier. The combination of a clinical management protocol like the one shown in Algorithm 57.1, with standing orders as shown in Table 57.1, will facilitate the implementation of a program for aggressive organ donor management. Multiple studies have shown that adherence to these aggressive management protocols will decrease the incidence of cardiopulmonary failure and increase the number of organs recovered per donor as well as the function of the transplanted organs.9,16–18 DuBose and Salim19 demonstrated that adherence to their aggressive management protocol (Fig. 57.1) was associated with an 82% increase in the number of donors, a 71% increase in the number of organs recovered, and an 87% decrease in the number of donors lost due to cardiopulmonary instability. In a separate report published by the Midwest Transplant Network in 2009,20 the number of OTPD was notably higher (p = 0.06) and the percentage of lungs transplanted was significantly higher (p < 0.01) among donors who underwent optimization by critical care providers. Finally, in an unpublished report from University of Pittsburgh Medical Center (UPMC), it was noted that use of critical care providers to support potential DCD donors in the prerecovery phase of the procurement process allowed these donors to progress to donation more comfortably and more quickly.
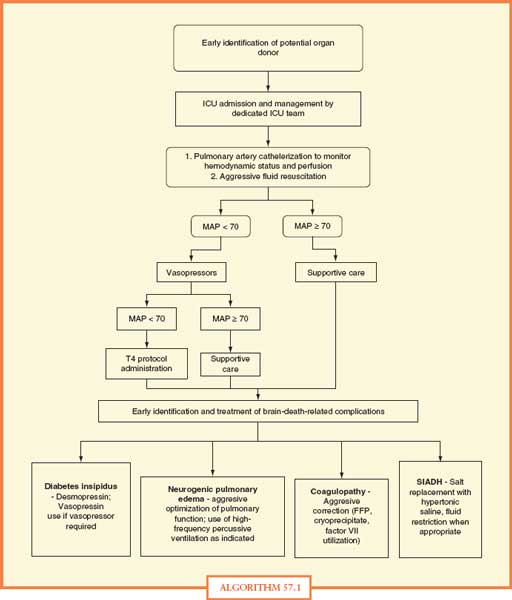
ALGORITHM 57.1 Standing orders for management of organ donors. Reprinted with permission from Shemie et al. Organ donor management in Canada: recommendations of the forum on Medical Management to Optimize Donor Organ Potential. CMAJ. 2006;174; S13–20, Appendix 3.
FFP, fresh-frozen plasma
ICU, intensive care unit
MAP, mean arterial blood pressure
SIADH, syndrome of inappropriate secretion of anti-diuretic hormone
T4, thyroxine
FIGURE 57.1. Organ donor clinical management protocol. Reprinted with permission from DuBose J, Salim A. Aggressive Organ Donor Management Protocol. J Intensive Care Med. 2008;23:367.
DMGs, donor management goals; OTPD, )organs transplanted per donor.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree