INTESTINAL OBSTRUCTION AND DYSMOTILITY SYNDROMES
Few clinical conditions are as common yet potentially perplexing as intestinal obstruction. Intestinal obstruction is a frequent presenting problem and accounts for a large percentage of surgical consults for acute abdominal pain. As many as 15% of surgical admissions are for mechanical small bowel obstruction.1
Intestinal obstruction develops when gas and succus are prevented from passing distally through the gastrointestinal tract as a result of either intrinsic or extrinsic compression (i.e., mechanical obstruction) or gastrointestinal paralysis (i.e., nonmechanical obstruction, either ileus or pseudo-obstruction). Many different etiologies may account for this pathology. Ileus is the most common form of dysmotility, although not an obstruction per se. It frequently develops after abdominal surgery or in association with intra-abdominal or extra-abdominal inflammatory conditions.2 Mechanical small bowel obstruction is slightly less common. Postoperative intra-abdominal adhesions account for the majority of mechanical small-bowel obstructions. Cancer and hernia account for the majority of the remaining cases of small-bowel obstruction. Mechanical colonic obstruction is less frequent than small-bowel obstruction, and accounts for 10%–15% of cases of mechanical bowel obstruction. The most common causes of mechanical large-bowel obstruction include obstructing carcinoma, diverticulitis, or volvulus. Acute colonic pseudo-obstruction occurs most often in elderly patients, in response to acute medical illness, or in the postoperative period.
PATHOPHYSIOLOGY OF INTESTINAL OBSTRUCTION
Mechanical small-bowel obstruction develops as gastrointestinal secretions and gas accumulate in the distended intestine proximal to an obstructed segment. As the distension progresses, the intraluminal hydrostatic pressure compresses the intestinal mucosal villus lymphatics, causing lymphedema of the bowel wall. With increasing intraluminal pressures, the hydrostatic pressure at the venous end of the postcapillary venules causes a shift in the Starling dynamics across the capillary bed, leading to the net filtration of fluids, electrolytes, and proteins into the bowel wall and lumen. This so-called “third spacing” can lead to the loss of massive amounts of intravascular volume, causing dehydration, hypovolemia, and end-organ hypoperfusion if untreated. Venous hypertension may also lead to arterial hypoperfusion and bowel ischemia in some cases, but, more often, direct arterial occlusion occurs due to the mechanical forces associated with a segment of bowel, which has twisted upon its mesentery (volvulus) or due to strangulation in an incarcerated hernia. If the bowel ischemia is not treated in a timely fashion, intestinal necrosis, perforation, and peritonitis ensue. Furthermore, the normal intestinal mucosal barriers are disrupted, theoretically allowing gut bacteria to translocate into the systemic circulation.3,4 Enteric bacteria have been isolated in the mesenteric lymph nodes of approximately 60% of patients undergoing laparotomy for simple bowel obstruction (without strangulated intestine) compared to only 4% of patients undergoing laparotomy for conditions other than bowel obstruction.5
DIAGNOSIS AND EVALUATION
Patients with mechanical bowel obstruction or ileus often present with abdominal pain and distension, nausea, vomiting, and obstipation.6 Several clinical features may help distinguish mechanical obstruction from ileus or pseudo-obstruction. The pain associated with mechanical obstruction is typically moderate to severe and crampy in nature, while patients with ileus tend to have less pain and in some cases no pain.
A detailed past surgical history must be obtained in all patients. Prior abdominal surgery places the patient at risk for the development of adhesive small-bowel obstruction. The lack of prior abdominal surgery or inflammatory conditions makes adhesive disease unlikely as the etiology of the patient’s obstruction. Any prior history of malignancy must be taken into account, as recurrent cancer must be in the differential diagnosis.
PHYSICAL EXAMINATION AND INITIAL MANAGEMENT
The patient with bowel obstruction often requires simultaneous evaluation and initial resuscitation. It is important to rapidly assess the degree of physiologic impairment, and address it expeditiously. Common physiologic abnormalities include hypovolemia, electrolyte abnormalities, and prerenal azotemia. Establishment of intravenous access and fluid resuscitation are important parts of the initial management of these patients. Careful monitoring of urinary output with an indwelling Foley catheter may serve as an important endpoint of resuscitation. Nasogastric decompression may be necessary to reduce the incidence of vomiting and aspiration. The character of the nasogastric tube output may be useful as far as diagnosis is concerned. A nonbilious output implies a gastric outlet obstruction. Bilious but nonfeculent aspirate is usually seen in proximal small-bowel obstructions or colonic obstruction with a competent ileocecal valve. Distal small-bowel obstruction often presents with feculent nasogastric output.
It is also important to exclude other medical conditions, which may be associated with nausea, vomiting, and abdominal distention such as an acute coronary syndrome or pneumonia.
Examination of the abdomen proceeds in an orderly fashion: inspection, auscultation, palpation, and percussion. With the patient in the supine position, general inspection of the abdomen is performed. The degree of abdominal distension may vary with the level of the obstruction: proximal small-bowel obstruction may be associated with minimal abdominal distension. Surgical scars from prior abdominal surgery should be noted. Visible peristaltic waves are often associated with mechanical small-bowel obstruction.
Auscultation with a stethoscope should be performed for at least 5 minutes to determine the presence and quality of bowel sounds. The typical bowel sounds in a patient with a mechanical small-bowel obstruction are high-pitched and hyperactive. Patients with ileus often have a silent abdomen. However, patients with long-standing intestinal obstruction or perforation associated with peritonitis may have a silent abdomen.
Palpation of the abdomen should start gently and become progressively deeper as the patient’s pain is assessed. If the patient complains of pain in one discrete region area, it is useful to begin the palpation on the opposite side of the abdomen. Most patients with bowel obstruction have diffuse abdominal tenderness, but less than half will have mild tenderness, guarding, or rigidity.6 Traditionally, many surgeons believe that the findings of localized tenderness, guarding, or rebound are indicative of underlying bowel strangulation and mandate operation. However, these findings are neither sensitive nor specific for the detection of underlying bowel ischemia7 or obstruction.6 Furthermore, patients with ileus may also have abdominal tenderness and may be difficult to distinguish from mechanical bowel obstruction in this regard. Gentle percussion should be performed to assess for tympany associated with underlying distended, gas-filled intestine; dullness, indicative of an underlying mass; or peritoneal irritation associated with ischemic bowel or peritonitis secondary to perforation.
A comprehensive evaluation for abdominal wall herniae must be performed in all patients. This includes careful inspection for inguinal, femoral, umbilical, incisional, and other abdominal wall herniae. Herniae should be assessed for the presence of incarcerated intestine. If local signs of infarcted intestine are present such as erythema or cellulitis, the patient should undergo immediate operation without attempt at reduction. Otherwise, careful reduction should be attempted, but care taken to avoid further injury to the intestine during this maneuver, or of reducing already ischemic bowel. Some herniae, such as obturator hernia or internal hernia, may not be readily detectable on physical exam and require imaging to detect. Digital rectal examination is performed to evaluate for masses, fecal impaction, and occult blood. Similarly, digital examination of any existing stoma is important to assess obstruction at a colostomy or ileostomy site.
DIAGNOSTIC STUDIES
Plain Radiographs
While abdominal radiographs should be obtained routinely on all patients suspected of having a bowel obstruction, plain films may be diagnostic in only half of such patients.8,9 Plain films are more sensitive in the detection of high-grade obstruction but less sensitive to detect low-grade obstruction. A chest x-ray helps to exclude an acute pulmonary process such as pneumonia, as well as detect subdiaphragmatic air, indicative of hollow viscus perforation. Plain abdominal x-rays (upright, lateral decubitus, and supine) can distinguish between mechanical bowel obstruction and ileus in many cases and may establish the location of the obstruction (small vs. large intestine). Except for inguinal hernia or gallstone ileus, the cause of the obstruction is often not discernable on plain radiographs. It is helpful to distinguish between gas-filled loops of small and large intestine. The small intestine contains valvulae conniventes or plicae circulares, which encompass the entire lumen of the bowel. Colon, which contains gas demonstrates colonic haustral markings, which cross only part of the bowel lumen (Fig. 36.1). Normally, the small intestine does not contain visible gas, so the finding of substantial gas in the small intestine is abnormal. The presence of air–fluid levels is also indicative of either mechanical obstruction or ileus. Gas throughout the small intestine and colon is usually associated with ileus, but can also be seen in cases of distal (rectal) obstruction. The presence of distended, air–fluid-filled loops of small intestine with absent colonic gas suggests high-grade small-bowel obstruction. However, in the early stages of small-bowel obstruction or in cases of partial obstruction, some gas may remain within the colon. Patients with closed-loop obstruction or very proximal small-bowel obstruction may have few or no dilated loops of intestine. Massive distension of the colon is seen in cases of pseudo-obstruction or colonic volvulus. Thickened intestinal walls with mucosal thumbprinting occurs when the intestine is edematous or ischemic (Fig. 36.1). Also, pneumatosis of the intestinal wall and portal venous gas result from advanced cases of intestinal ischemia. Free intraperitoneal air indicates perforation of a hollow viscus.
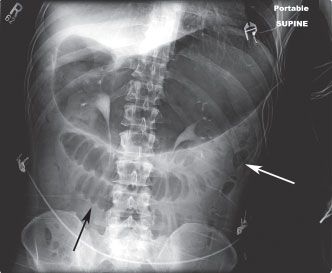
FIGURE 36.1 Abdominal radiograph of a supine patient with small-bowel obstruction. The stomach is markedly distended. There are several dilated small-bowel loops, which demonstrate prominent valvulae conniventes with bowel wall edema (black arrow). Scant gas is seen within the decompressed colon (white arrow).
Laboratory Studies
Patients with mechanical bowel obstruction often have fluid and electrolyte disturbances that should be corrected during the resuscitation phase and prior to surgical intervention. Likewise, patients with ileus often have an associated metabolic or infectious etiology that should be sought as part of the diagnosis and treatment of the condition. Serum electrolytes, creatinine, hemoglobin, and coagulation parameters should be checked routinely. Serum lactate may be elevated in cases of bowel ischemia. Patients with considerable physiologic impairment may need assessment of acid–base status with arterial blood gas. For patients with ileus, additional studies that should be obtained include serum magnesium, phosphate, ionized calcium, urinalysis, pancreatic enzymes, and a search for potential infectious sources.
Computed Tomography
Many clinicians rely on computed tomography (CT) to determine the etiology and location of intestinal obstruction,10–14 and CT has become the radiographic modality of choice for the diagnosis of intestinal obstruction.9 The diagnosis of small-bowel obstruction on CT involves the identification of dilated loops of intestine proximally with normal or collapsed loops distally. A small-bowel caliber > 2.5 cm is considered dilated. If a transition point is identified, the diagnosis of obstruction is more certain. The transition point often resembles a bird’s beak (Figs. 36.2 and 36.3), and this “beak sign” is present in 60% of cases of small-bowel obstruction.9 The small-bowel feces sign, the presence of small-bowel particulate material in a dilated segment (Fig 36.4), is present in 56% of cases of small-bowel obstruction.15 CT has a sensitivity of 81%–94% and specificity of 96% for diagnosis of high-grade bowel obstruction.9 The accuracy of CT scan is reduced in cases of partial intestinal obstruction, although recent advances in CT technology are improving its accuracy in these cases. CT has several advantages over other imaging modalities: it can accurately determine the level, etiology, and degree of the obstruction and can readily indentify closed-loop obstruction and bowel ischemia. CT can detect extrinsic mass lesions or inflammatory processes not visible on plain radiographs.16 CT is the most sensitive modality to detect intraperitoneal free air and pneumatosis intestinalis (Fig. 36.4C). CT is also the preferred modality to distinguish mechanical colonic obstruction from pseudo-obstruction.17 Administration of oral contrast may not be tolerated by acutely ill and obstructed patients and is usually not essential for the CT identification of obstruction; luminal fluid and air can easily be distinguished within the bowel lumen. However, enteral contrast may be useful in discriminating partial from complete obstruction and the level of the obstruction. The use of intravenous contrast during CT is recommended so the bowel wall can be visualized in contrast to its luminal contents.18–20 The most important information that CT can provide is whether strangulation (bowel ischemia) is present. The sensitivity of contrast-enhanced CT for diagnosis of intestinal ischemia is as high as 90%.9 Signs of intestinal ischemia on contrast-enhanced CT include thickened bowel wall, ascites, the target sign (trilaminar appearance of bowel wall from enhanced mucosa and muscularis with edematous submucosa in between), lack of contrast enhancement of bowel wall, pneumatosis intestinalis, gas in mesenteric or portal veins, and the “whirl sign” (twisting of mesenteric vessels in volvulus).
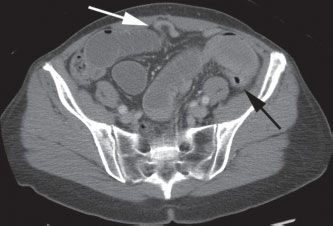
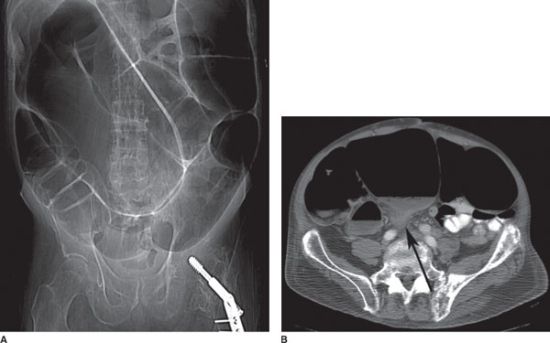
FIGURE 36.2 CT demonstrating high-grade small-bowel obstruction with a transition point or “bird’s beak” (white arrow) between distended and decompressed loops of small intestine. The decompressed descending colon is also seen (black arrow).
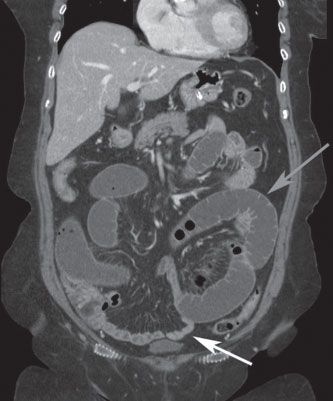
FIGURE 36.3 Coronal CT scan demonstrating small-bowel obstruction with dilated proximal small intestine (gray arrow) and decompressed distal small intestine (white arrow) beyond the level of the obstruction.
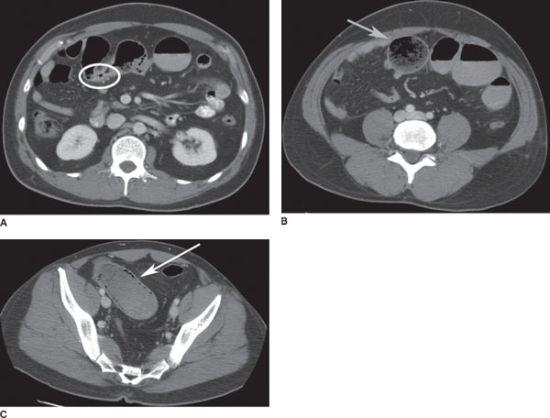
FIGURE 36.4 A: CT scan showing small-bowel obstruction with transition point at a focal area of wall thickening (white ellipse). B: proximal to the obstruction, there is marked dilation of the small bowel with small-bowel feces sign (gray arrow). C: pneumatosis intestinalis of a segment of ischemic, obstructed small intestine (white arrow).
Other Radiographic Modalities
Given the limitations of plain abdominal radiographs described above, other adjunctive radiographic modalities have been advocated. Clearly, CT is the modality of choice given its availability and accuracy. However, in some instances, ultrasonography and magnetic resonance imaging (MRI) have been proposed. Both modalities are highly sensitive and specific for intestinal obstruction when performed and interpreted by experienced clinicians. Two prospective trials found that ultrasonography was as sensitive as and more specific than plain radiographs in the diagnosis of intestinal obstruction.21,22 The accuracy of ultrasonography is operator dependent, and most surgeons are not yet comfortable in the interpretation of sonographic images for the diagnosis of intestinal obstruction. These reasons, plus the accuracy and widespread availability of CT, limit the clinical utility of ultrasonography in this setting. However, for patients who are hemodynamically unstable and not suitable candidates for transport to CT, bedside ultrasonography may be a useful modality.21
There is evidence suggesting that MRI is more sensitive and specific than contrast-enhanced CT in determining the etiology and location of bowel obstruction.23 However, continuing evolution and technological advances in CT imaging, such as multiphasic scanning, faster scanners, and 3D reconstruction, have increased the accuracy of this modality, further reducing the utility of ultrasonography and MRI.
Gastrointestinal contrast studies may be useful diagnostic modalities in some instances, but best used in the subacute or chronic setting. Oral contrast studies such as small-bowel follow-through can offer information about the location and degree of the obstruction and the bowel transit time. Limitations of the small-bowel follow-through include logistics, the length of time required to complete the study, the dilution of contrast as it travels distally, and the risk of contrast aspiration. Enteroclysis allows nondistensible segments of intestine to be more readily identified. Enteroclysis is performed by placing a catheter in the small intestine and infusion of contrast material. Enteroclysis is accurate at detection of low-grade and intermittent obstruction and can serve as an adjunct to CT in these cases; it has no advantage over CT in the diagnosis of high-grade obstruction.9 The administration of contrast enemas may be useful in cases of colonic distension to evaluate for mechanical obstruction, for example, due to mass lesions or stricture.
Sigmoidoscopy
For patients with distal colon or rectal obstruction, sigmoid volvulus, or colonic pseudo-obstruction with massive colonic distension, flexible or rigid sigmoidoscopy may be both diagnostic and therapeutic. When the radiographs demonstrate distended colon with gas extending to the sigmoid or rectum, sigmoidoscopy will readily exclude a distal colon or rectal mass as the etiology of the obstruction. Care must be exercised to avoid instillation of a large amount of air during the sigmoidoscopy; this will increase the chances of iatrogenic perforation of the colon.
DETERMINATION OF THE NEED FOR SURGERY
For patients with suspected bowel obstruction, the decision to operate and the timing of surgery can be determined based on the history, physical exam, laboratory data, plain radiographs, and CT. It is important to distinguish mechanical obstruction from ileus. Patients with nonmechanical obstruction usually do not require immediate surgery. Early identification of patients with obstruction and bowel ischemia is critical. Also, in patients with mechanical obstruction, determination of whether the obstruction is complete (which requires immediate operation) vs. partial (which does not) is important. Similarly, determination of the level and etiology of the obstruction has important therapeutic implications. Patients with complete or partial bowel obstruction generally should be admitted to a surgical service as admission to nonsurgical service is associated with increased patient morbidity and mortality.24,25 In one series of 166 cases of small-bowel obstruction, 20 patients underwent immediate surgery due to concern for bowel ischemia and 45% of these proved to have ischemic bowel at laparotomy. Among those selected for conservative management, about two-thirds resolved without surgery, but 6% had strangulated bowel and 2% died.26
MECHANICAL OBSTRUCTION
There are many potential causes of bowel obstruction (see Tables 36.1 and 36.2). Patients with mechanical, complete bowel obstruction should undergo immediate surgery after expeditious correction of hypovolemia and fluid and electrolyte disorders. Nonoperative management of complete intestinal obstruction is associated with increased morbidity and mortality due to delayed recognition and treatment of strangulated bowel.7 Immediate operation is also indicated for patients with bowel obstruction in the presence of peritonitis or signs of systemic toxicity, incarcerated or strangulated herniae, pneumatosis intestinalis, cecal volvulus, or sigmoid volvulus with systemic toxicity. CT will help identify the presence of these conditions in equivocal cases. Exceptions to this rule for immediate operation for these conditions include patients with terminal illness whose goals of care are palliation of symptoms, or those who have cardiopulmonary instability, which requires resuscitation prior to surgery.
TABLE 36.1
ETIOLOGY AND INCIDENCE OF SMALL-BOWEL OBSTRUCTION
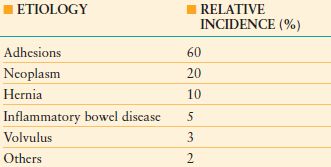
From Hayanga AJ, Bass-Wilkins K, Bulkley GB. Current management of small-bowel obstruction. Adv Surg. 2005;39: 1–33
TABLE 36.2
ETIOLOGY AND INCIDENCE OF LARGE-BOWEL OBSTRUCTION
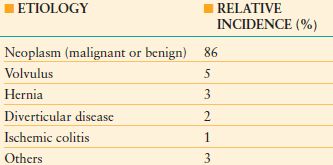
From Biondo S, Pares D, Frago R. et al. Large-bowel obstruction: predictive factors for postoperative mortality. Dis Colon Rectum. 2004;47: 1889–1897.
ADHESIVE SMALL-BOWEL OBSTRUCTION
Postoperative adhesions are the most common cause of intestinal obstruction and account for 60% of cases of small-bowel obstruction.29 Adhesive intestinal obstruction usually occurs in the small intestine. In the setting of colonic obstruction, an alternative etiology should be sought as adhesions rarely cause colonic obstruction. Intestinal obstruction resulting from adhesions may occur as early as days to as late as many years after surgery.30 Adhesive small-bowel obstruction most frequently occurs after prior appendectomy, colorectal surgery, gynecologic procedures, or upper gastrointestinal surgery. One-fourth of patients with mechanical small-bowel obstruction had multiple prior laparotomies.29 Laparoscopic surgery is associated with a lower incidence of postoperative bowel obstruction compared to comparable open surgery.31 Initial treatment of adhesive small-bowel obstruction includes bowel rest, nasogastric decompression, intravenous fluids, correction of metabolic and electrolyte abnormalities, and analgesia. In this setting, nonoperative management is associated with resolution of symptoms in approximately 90% of patients.32–34 However, as many as half of these patients will experience recurrent obstruction.34–36
Some studies suggest that the probability of resolution of the bowel obstruction with conservative management can be predicted based on the nature of the prior abdominal surgery.37–39 Procedures associated with a higher failure rate for conservative management of adhesive small-bowel obstruction include those which were performed through a midline laparotomy, those involving the aorta, colon, rectum, appendix or pelvic adnexa, and those done to alleviate previous obstruction due to carcinomatosis. In these cases, a shorter duration of observation during conservative management should be considered.
For patients with partial bowel obstruction due to adhesions, there is no clear consensus on how long such patients should be treated conservatively. However, beyond 48 hours of observation, the risks of complications increase substantially and the probability of nonoperative resolution decreases considerably.40 In general, patients who are likely to resolve with conservative management begin to show clinical improvement in the first 12 hours. Consideration for surgical intervention should be made for patients who fail to show signs of improvement or who deteriorate in this time frame. Patients selected for conservative management must be examined serially and frequently, preferably by the same clinician. The degree of abdominal distension, amount and character of the nasogastric output (e.g., feculent output correlates with complete obstruction, necessitating surgery), the passage of flatus or bowel movement, and the development of new or worsening abdominal tenderness should be carefully assessed at frequent intervals. Follow-up plain radiographs may be useful to demonstrate persistence or resolution of the radiographic abnormality.
It should be possible to determine with a high degree of accuracy which patients will require surgery to address adhesive small-bowel obstruction within 24–48 hours of admission. Using CT, patients with complete bowel obstruction or closed-loop obstruction, that is, those who require urgent operation, can be readily identified and treated appropriately.11–13 For those with partial obstruction, close clinical observation will identify those who are failing to progress and warrant surgical intervention. In addition, the success or failure of nonoperative management can be predicted by the time it takes for orally administered contrast to reach the right colon. Arrival of contrast within 8–24 hours predicts success of conservative management with >95% sensitivity and specificity.41–43
SURGICAL ADHESIOLYSIS
Laparotomy with surgical adhesiolysis is the traditional surgical modality to treat patients with adhesive bowel obstruction who require operative intervention. The principles of this technique involve midline laparotomy with careful entry into the peritoneal cavity to avoid iatrogenic bowel injury, identification and sharp lysis of the adhesions that contribute to the obstruction, careful inspection of the intestine to identify injury or ischemia, repair or resection of perforated intestine, and resection of ischemic or gangrenous segments. When operative adhesiolysis is required for small-bowel obstruction, the mortality ranges from <5% for uncomplicated obstruction, to as high as 30% in cases where bowel resection is required for intestinal strangulation or gangrene.30
Laparoscopic lysis of adhesions relieves obstruction in the majority of patients and compares favorably to open surgery.44–49 To reduce the chance of bowel injury during trocar placement, the first port should be placed using open technique under direct visualization and in an area away from prior surgical incisions.50,51 In retrospective, case-control matched series, about half of patients treated with laparoscopic adhesiolysis required conversion to open surgery due to inability to complete the procedure laparoscopically or to manage complications.49 Patients with two or more prior laparotomies had a considerably higher rate of intraoperative complication during laparoscopy. Despite the high conversion rate, the group of patients who underwent laparoscopic adhesiolysis experienced an overall reduction in postoperative complications. Other studies have also shown that patients explored laparosopically who required conversion to open laparotomy did not fair worse than those initially treated with laparotomy.52
One potential advantage of laparoscopic surgery for adhesive bowel obstruction over open surgery is that it results in a reduced risk of causing additional intra-abdominal adhesions, which may lead to subsequent adhesive bowl obstruction.53,54 However, patients who undergo laparoscopic surgery for adhesive bowel obstruction may be at increased risk for early unplanned reoperation due to complications or incomplete relief of obstruction.44 Another problem is that iatrogenic bowel perforation during laparoscopic adhesiolysis may not be detected during the initial surgery.50 Thus, a low threshold to convert to open surgery is advocated when laparoscopic adhesiolysis fails to identify and treat an obvious point of obstruction or when the adhesiolysis is difficult or unsafe. Preemptive conversion to open laparotomy when there is poor visualization or dense adhesions is preferable to reactive conversion after an iatrogenic injury has occurred.55 In addition, careful patient selection is also important; the laparoscopic approach may best be suited for patients who have undergone one or two prior abdominal operations, especially if they have undergone appendectomy only, and in whom the etiology of the obstruction is felt to be adhesive bands.56 Patient selection for laparoscopic adhesiolysis is important. Indications for laparoscopic exploration include proximal obstruction, partial obstruction, anticipated single band, localized radiographic distension, and mild abdominal distension. Contraindications to laparoscopy for bowel obstruction include coagulopathy, inability to tolerate general anesthesia, severe abdominal distension, massively dilated loops of intestine, peritonitis, sepsis, hemodynamic instability, and dense adhesions with fused loops of intestine or multiple prior laparotomies. The surgeon’s experience and advanced laparoscopic skills may also be an important factor in determining the safety and efficacy of laparoscopic adhesiolysis for bowel obstruction.57,58
INTESTINAL OBSTRUCTION IN THE EARLY POSTOPERATIVE PERIOD
A common surgical dilemma is the patient who develops abdominal distension, obstipation, nausea, vomiting, and pain early after abdominal surgery. It may be difficult to differentiate postoperative ileus from mechanical small-bowel obstruction in this patient population. As many as 10% of postoperative patients develop mechanical small-bowel obstruction,59 and in 90% of these cases, the etiology is adhesive disease.60,61 In most cases, plain abdominal radiographs will help differentiate between ileus and mechanical bowel obstruction in this patient population.60
Furthermore, it is imperative to exclude technical complication as the etiology of the patient’s postoperative ileus or obstruction. Complications such as anastomotic leak, abscess, internal hernia, perforation, anastomotic stricture, or stomal obstruction must be identified and treated appropriately. These conditions are unlikely to resolve with nasogastric decompression and bowel rest. When plain abdominal radiographs are unrevealing, CT is the appropriate study in this situation.
In most patients without peritoneal irritation or systemic toxicity, intestinal obstruction in the early postoperative period can be managed safely with bowel rest, intravenous fluids, and nasogastric decompression.59,60 Because the risk of intestinal strangulation in patients with early postoperative adhesive obstruction is lower (<1%)60,61,62 than those who present with obstruction in a delayed fashion, these patients are usually managed conservatively for longer periods of time. Close to 90% of patients resolve spontaneously after 2 weeks of conservative management. Approximately 70% of those who will resolve with nonoperative management do so in the first week, with another 25% responding by the second week. Beyond 2 weeks, patients with persistent obstruction are unlikely to resolve spontaneously and should undergo operation to manage the obstruction.60,61 Parenteral nutritional support should be initiated in patients who will be treated conservatively and with bowel rest and no enteral nutrition for >7–10 days.63 In the past, long intestinal tubes were advocated to manage postoperative bowel obstruction.33 However, a randomized trial of long intestinal tubes versus standard nasogastric tubes found no difference in time to resolution of obstruction, the need for operation, or the duration of postoperative ileus.64
BOWEL OBSTRUCTION IN PATIENTS WITHOUT PRIOR ABDOMINAL SURGERY
While adhesive disease is the etiology of intestinal obstruction in most patients with prior abdominal surgery, patients who have never undergone surgery and who develop intestinal obstruction should be carefully evaluated for an underlying etiology that requires surgical intervention. Such patients who have partial obstruction can be appropriately admitted for conservative management during this evaluation. These patients often have an external or internal hernia, tumor, malrotation, volvulus, or intussusception. Malignant tumors account for 20% of small-bowel obstructions, and is the most common cause of small-bowel obstruction after postoperative adhesions.65 The most commonly utilized first-line test is CT, which will identify the etiology in the majority of cases. Once the obstruction resolves and if CT is unrevealing, a small-bowel follow-through may provide a diagnosis. In some cases, elective exploratory laparoscopy or laparatomy may be necessary to diagnose and treat the underlying etiology.
STRANGULATION AND CLOSED-LOOP OBSTRUCTION
The type, location, and etiology of intestinal obstruction will impact the likelihood of resolution without surgery as well as the morbidity and mortality. Strangulation occurs when blood supply to a segment of intestine is compromised, usually due to a loop of intestine trapped in an abdominal wall or internal hernia, a volvulus or intussusception. Venous flow is usually affected first, leading to bowel wall edema, which first affects the mucosa and submucosal layers. Decreased arterial flow and pressure to the affected segment follows. These factors contribute to reduced blood flow to the segment of intestine and increased permeability and edema of the involved bowel. Substantial fluid sequestration and systemic hypovolemia can result. As the local process progresses, inadequate blood flow leads to release of inflammatory mediators, the potential for bacterial translocation, intestinal gangrene, and perforation with peritonitis.
Strangulation occurs in approximately 10% of patients with mechanical small intestinal obstruction. While the mortality associated with uncomplicated intestinal obstruction is <5%, when strangulation develops, mortality increases to 10%–37%.7,40,66 The goal of surgical intervention is to relieve the obstruction prior to the development of intestinal strangulation to reduce the associated morbidity and mortality. Patients at highest risk for strangulation are those who present with an incarcerated hernia, volvulus, closed-loop or complete intestinal obstruction. Early identification of these processes by careful physical exam and radiographs (plain abdominal radiographs or CT) is paramount to timely surgical management and are indications for immediate surgery. Unfortunately, physical exam alone has a poor sensitivity to detect early signs of obstruction with strangulation,7 prompting many clinicians to recommend routine use of CT for patients with mechanical bowel obstruction admitted for a trial of conservative management. Contrast-enhanced CT can demonstrate compromised blood supply and intestinal wall edema in early cases of intestinal strangulation.14,67,68 Radiographic evidence of pneumatosis intestinalis and free intraperitoneal air are late findings of gangrene and perforation, and indications for immediate operation; but the goal should be to intervene prior to the development of these findings.
Closed-loop obstruction is a specific type of obstruction in which two points along the course of the bowel are obstructed at a single location, thus forming a closed loop. Usually this is due to adhesions, volvulus, or internal herniation. Closed-loop obstruction is more likely to cause intestinal ischemia than obstruction due to simple adhesive disease, but may also be more difficult to diagnose on plain radiographs. CT often demonstrates a “whirl sign.”69
INTESTINAL OBSTRUCTION DUE TO HERNIA
Hernia is the etiology of 10% of cases of small-bowel obstruction, and is more likely to be associated with strangulation than obstruction due to adhesions.7,70 For patients without a history of prior abdominal surgery, hernia is the second leading cause of intestinal obstruction after neoplasm. Any hernia that is incarcerated, tender to palpation, is associated with skin changes such as erythema or induration, is an indication for immediate surgery. Ultrasonography and CT are appropriate diagnostic modalities in cases where the hernia is difficult to detect on physical examination.
Paraduodenal hernia, which is a congenital abnormality resulting from intestinal malrotation, has been recognized as an important cause of closed-loop obstruction in adults.71,72 It may account for as many as half of internal herniae. Patients present with a spectrum of symptoms ranging from mild abdominal discomfort and nausea to catastrophic closed-loop obstruction with intestinal gangrene and perforation. The diagnosis is readily made by CT or upper gastrointestinal contrast study, and operation should be performed expeditiously to prevent strangulation and peritonitis.71
VOLVULUS
Intestinal volvulus is a closed-loop obstruction caused by twisting of the intestine on its mesentery, leading to obstruction and impaired blood supply to the segment of intestine. Patients present with acute colicky abdominal pain, nausea, vomiting, and distension. Small-bowel volvulus may not be readily apparent on abdominal radiographs because the closed-loop fills with fluid and air–fluid levels may be absent, but the fluid-filled loop is usually apparent on CT. The finding of small-bowel volvulus mandates surgical intervention.
Colonic volvulus can occur anywhere in the large intestine, but is most common in the sigmoid (Fig. 36.5) followed by the cecum. Plain abdominal radiographs are often diagnostic, usually making CT unnecessary. Patients with colonic volvulus who have signs of systemic toxicity, signs of peritoneal inflammation on physical examination, or bloody rectal output require immediate operation. For patients with sigmoid volvulus, in the absence of these indications for immediate surgery, endoscopic decompression is the most appropriate first-line treatment and is effective in approximately 95% of patients.73 During sigmoidoscopy, careful navigation of the sigmoidoscope with minimal air insufflation should be performed to reduce the risk of perforation. The finding of mucosal gangrene during sigmoidoscopy is an indication to abort the procedure and proceed immediately for surgery. Plain abdominal radiographs should be obtained after the endoscopic decompression to exclude the possibility of perforation. After successful endoscopic decompression, the patient should be evaluated and prepared for definitive surgical therapy during the same hospital admission since the likelihood of recurrent volvulus is high; sigmoidectomy with primary anastomosis is the preferred operation in suitable candidates. Patients with colonic volvulus proximal to the sigmoid colon usually require surgical intervention; endoscopic decompression is usually unsuccessful and associated with a considerably higher rate of complications.
FIGURE 36.5 A: Abdominal radiograph of a patient with sigmoid volvulus, demonstrating massively distended sigmoid colon, which extends from the left lower quadrant to the right upper quadrant. B: CT scan of the same patient demonstrates a “beak sign” (black arrow) where the sigmoid colon twists upon itself, resulting in complete obstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree