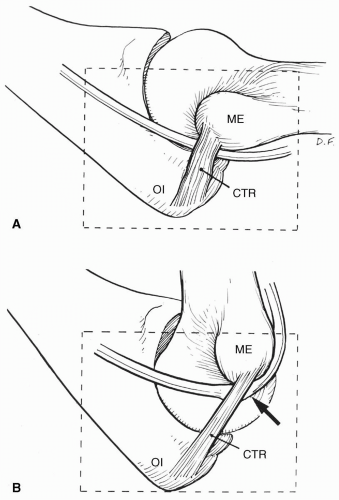
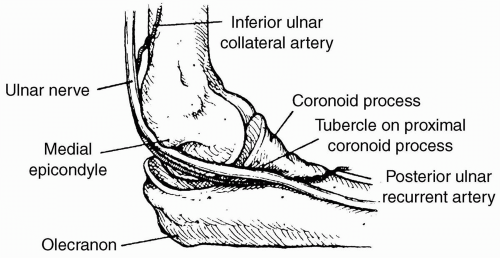
Are Perioperative Nerve Injuries Always Preventable?
Perioperative ulnar neuropathy may occur despite the use of extensive arm and/or elbow padding during surgery and even with “proper positioning of the arms.” There are no concrete data to support or recommend one type of padding over another (such as gel padding vs. foam padding). In addition, it is possible that a given placement or type of padding may increase direct pressure on a peripheral nerve. An analysis of perioperative nerve injuries detected by the American Society of Anesthesiologists (ASA) Closed Claims Database revealed that ulnar nerve injuries accounted for 34% of 227 perioperative nerve injuries.
12 However, the mechanism of injury was clearly determined in only 10 (9%) of the 113 cases of ulnar nerve injuries. Of the 10 cases where a mechanism of injury was determined, 3 were associated with the performance of an axillary block, four were attributed to preoperative trauma, one was because of intraoperative trauma, one was caused by the use of crutches, and one resulted from the surgical procedure. Difficulty in determining specific mechanisms of injury is further amplified by the finding that ulnar nerve injury occurs with nearly equal frequency for both medical and surgical patients hospitalized for >2 days
13,
14 (see
Table 57.3, for overlapping confidence intervals). Men are predisposed to ulnar nerve injury likely because of gender-based anatomic variations of the cubital tunnel. Prolonged periods of bed rest in the supine position—whether during or after surgery or for medical conditions—may contribute to the etiology of perioperative ulnar nerve injury, particularly in men.
13,
14,
15Most perioperative ulnar nerve injuries are not evident immediately after surgery. In fact, the ASA Closed Claims Database review demonstrated that only 21% of cases were evident in the immediate postoperative period, although 62% became evident between 1 and 28 days, with a median of 3 days.
12 Along with the similar incidence of ulnar nerve injury in hospitalized nonsurgical patients, this delayed presentation raises the question of when a “perioperative” nerve injury actually occurs. Therefore, it is often difficult to determine if an injury occurred intraoperatively, in the PACU, or at some time after the patient returned to the ward or to home.
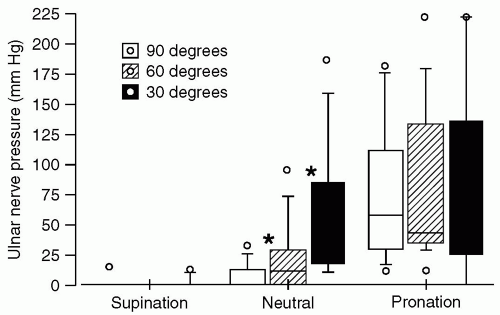
These confounding factors notwithstanding, it is important to minimize direct pressure on the ulnar nerve, particularly pressure exerted by noncompliant or rigid surfaces. When a supine individual has an arm abducted on an armboard, research data indicate that direct pressure against the ulnar groove is minimized by having the forearm supinated, with the palm up. Pronation results in the greatest pressure against the ulnar groove, although the neutral position was intermediate.
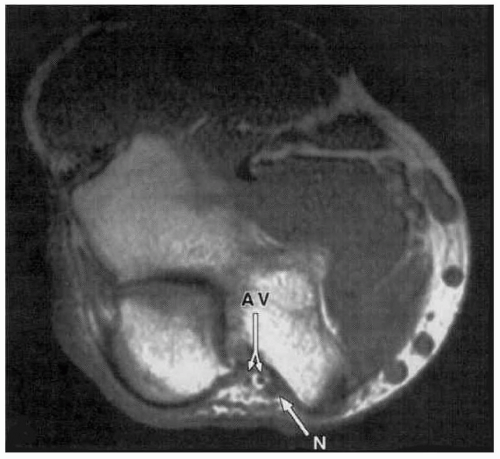
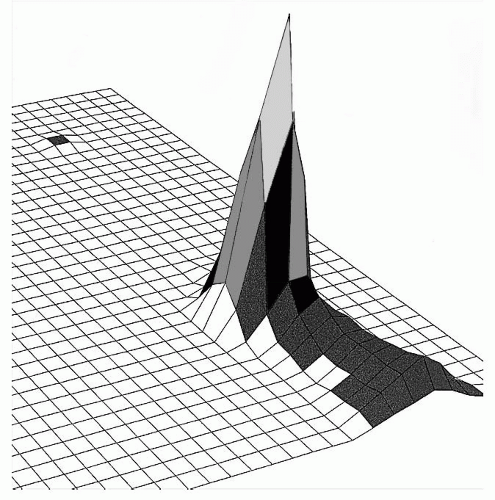
16 It is important to note that these data pertain to pressure only and do not address stretch. In addition, abduction to 90 degrees results in less direct pressure than abduction to 30 or 60 degrees. These data were obtained using a pressure-sensing pad that detected surface pressure distribution beneath the ulnar nerve with 1 cm
2 resolution (see
Fig. 57.8). Forearm supination minimized direct pressure exerted directly upon the ulnar nerve, because this position decreased contact with the weight-bearing surface (see
Table 57.4 and
Fig. 57.9). Conversely, pressure localized over the ulnar nerve was
greatest with the forearm pronated. Indeed, with the forearm in supination, only 6 of 50 subjects manifest
any pressure directly upon the ulnar nerve. With the forearm in neutral orientation, pressure over the ulnar nerve decreased as the arm was abducted from 30 to 90 degrees (see
Fig. 57.10).
In cases where SSEP monitoring is being performed (such as for spinal cord surgery), the ulnar nerve may be monitored as an upper extremity control and may also provide information for positioning purposes. Interpretation of abnormalities generally requires good communication between the monitoring neuroelectrophysiologist, the anesthesia team, and the surgeon. Deepened levels of anesthesia, hypothermia, hypotension, and anemia may cause global increases in SSEP latency or decreases in SSEP amplitude. In cases where abnormalities are detected, assessment and decision making should consider global signals, surgical manipulation, mechanical factors, electric factors, and/or positioning.
Figure 57.11 demonstrates the focal changes that occurred in a 62-year-old male patient undergoing an anterior cervical discectomy and fusion, during which the anesthesia level remained constant and unilateral decreases in ulnar SSEP amplitude were seen. Lower extremity SSEP signals were unchanged, making it unlikely that the SSEP changes were due to depth of anesthesia or global monitoring effects. The arm (which was previously tucked and padded with gel foam at the patient’s side) was therefore repositioned. The blood pressure cuff on that arm was also moved to the forearm, below the elbow. Within a few minutes, ulnar nerve SSEP amplitude began to recover. The patient was awakened at the conclusion of the operation and was neurologically intact with no ulnar nerve deficit throughout his postoperative course. While it is neither necessary nor recommended—nor practical—to use sophisticated neuroelectrophysiologic monitoring on all patients undergoing anesthesia and surgery, on those in whom this modality is utilized, unique information may be obtained regarding intraoperative nerve dysfunction, on a case-by-case basis.
RECENT SCIENTIFIC INVESTIGATIONS AND ANESTHETIC IMPLICATIONS
Relatively recent studies have tested assumptions regarding the etiology of ulnar neuropathy
16,
17,
18 using quantitative and physiologic models of ulnar nerve stress. One such study characterized the ulnar nerve response to various experimental stressors (stretch, pressure, and ischemia), which might be encountered in the preoperative setting.
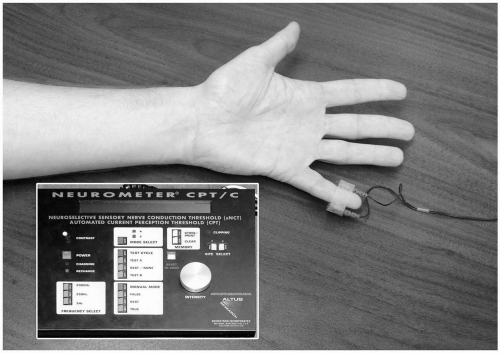
18 Alterations in current perception threshold (CPT) were used as a surrogate marker of ulnar nerve dysfunction (see
Fig. 57.12). CPT analysis also allowed the differentiation between nerve fibers subtypes. Nerve ischemia produced with an arm tourniquet inhibited all three fiber subtypes. Conversely, a model of ulnar nerve stretch (produced by arm flexion at the elbow to 110 degrees) failed to produce significant CPT increases at any of the three stimulating frequencies. However, direct pressure over the ulnar nerve produced significant CPT increases at 5 Hz and 250 Hz, indicating inhibition of both unmyelinated C fibers and myelinated Aδ fibers. In addition, C fibers demonstrated significant gender differences, with nerve pressure having a 1.7-fold (95% confidence interval, 1.2- to 2.4-fold) greater effect in men (see
Table 57.5). This 70% increase in C-pain fiber susceptibility to direct pressure in men could be a partial explanation for the threefold greater frequency of perioperative ulnar neuropathies in men.
Lastly, in a comparison of the onset of clinical paresthesia to the onset and severity of SSEP electrophysiologic changes, intentional ulnar nerve compression was induced in 16 male volunteers by placing a wooden dowel snugly in the ulnar groove and allowing the full weight of the arm to rest directly on the wooden block for a maximum of 60 minutes, while recording maximal decreases in SSEP waveforms.
16 Eight subjects complained of a progressive hand paresthesia 37 minutes after placement of the wooden block in the ulnar groove, and all eight of these subjects also manifested significant SSEP changes with a mean decrease in the N9-N9N amplitude of −44% (range of −20 to −71%). By contrast, eight volunteers reported
no ulnar paresthesia during 60 minutes of a similar pressure from the wooden block in the ulnar groove. Nevertheless, these eight subjects demonstrated a mean SSEP decrease in the N9-N9N waveform amplitude of -44% (range of -19% to -72%) (see
Table 57.6). These results suggest that up to one half of male patients who experience pressure on peripheral nerves sufficient to impair electrophysiologic function may be “at risk” because they
do not perceive a concurrent paresthesia of that ulnar nerve. Therefore, significant ulnar nerve compression and dysfunction can occur in unsedated men in the
absence of perceived symptoms.