Cardiac Dysrhythmias
Angela T. Truong
Ann T. Tong
Dam-Thuy Truong
Marc A. Rozner
CASE SUMMARY
A 54-year-old man (90 kg, 181 cm, body mass index [BMI] 27.7 kg per m2) presented for wide local excision of a melanoma on his thigh, along with intraoperative lymphatic mapping and sentinel lymph node biopsy. He had no medical allergies, took no medicines, and reported excellent exercise tolerance (he walks his treadmill at 5 mph and 15% grade for 30 to 60 minutes nearly every day). His preoperative examination was significant only for sinus bradycardia with a rate of 54 bpm. His heart and lung examination findings were normal. His laboratory values (the surgeon ordered a complete blood count, electrolytes, blood urea nitrogen [BUN], creatinine, and lactic dehydrogenase) and electrocardiogram (ECG) demonstrated no abnormalities.
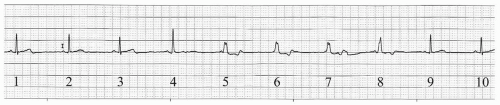
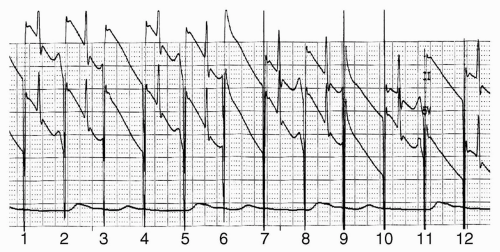
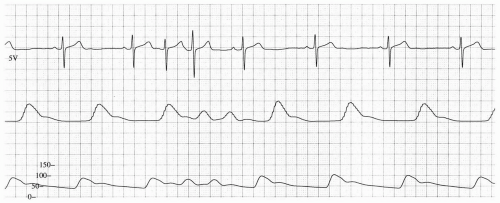
Anesthetic induction was carried out with fentanyl 200 µg, propofol 200 mg, and cisatracurium 14 mg. His trachea was intubated with a 7 Fr endotracheal tube, and anesthesia was maintained with desflurane 5.8% in 50% oxygen per air. Cefazolin (1 gm) was given. Shortly after anesthetic induction, but before incision, his sinus rate fell into the 40s and he developed isorrhythmic atrioventricular dissociation (see Fig. 18.1). His blood pressure (which had been 120/50 mmHg) fell to 80/30. A decision was made to insert a transesophageal pacemaker (TAPSCOPE, Cardiocommand, Inc, Tampa, FL), and pacing was begun at 60 bpm. When the pacing rate was increased to 90 bpm, the patient developed a second degree, Mobitz type I (Wenckebach) block (see Fig. 18.2).
After completion of his surgery, he admitted to feeling occasional “pounding” in his chest and neck, especially at night. A subsequent Holter monitor revealed significant sinus bradycardia with ventricular escapes. A permanent pacemaker was suggested to increase his overall heart rate, because his postoperative echocardiogram showed moderate aortic regurgitation in a structurally normal valve.
What Is the Importance of Cardiac Dysrhythmias?
Cardiac dysrhythmia (also arrhythmia) comprises any abnormality or perturbation in the normal activation sequence of the myocardium. Cardiac dysrhythmias can produce too slow a ventricular rate (bradydysrhythmia) or too fast a ventricular rate (tachydysrhythmia). These abnormalities frequently occur in the perioperative period. Although some are benign and require only watchful waiting or assurance of no biochemical derangements, others result from developing or ongoing malignant process(es). Some dysrhythmias represent a harbinger of a more serious condition (e.g., bradycardia that develops in the face of arterial hypoxemia). According to Atlee, the first recorded death during anesthesia, that of Hannah Greener in 1848,1 was most likely because of ventricular fibrillation (VF) (a malignant cardiac dysrhythmia) resulting from the “sensitizing” action of chloroform.2
Although lethal cardiac dysrhythmias remain a rare occurrence, any abnormal cardiac rhythm represents a potentially unstable condition. Some dysrhythmias are dangerous because they provoke inappropriate medical intervention (such as the treatment of benign premature ventricular contractions with antiarrhythmic agents as documented in the Cardiac Arrhythmia Suppression Trial [CAST] study3), whereas other abnormal rhythms can threaten cardiovascular homeostasis. Both bradydysrhythmias and tachydysrhythmias can produce an imbalance between myocardial oxygen supply (by reducing cardiac output or shortening diastole) and demand (by increasing rate), and some dysrhythmias can progress to life-threatening situations (e.g., supraventricular tachycardia producing myocardial ischemia, leading to ventricular tachycardia [VT] and death).
A cardiac dysrhythmia should always be considered in the differential diagnosis of any sudden hemodynamic imbalance. For example, an abrupt reduction in blood pressure associated with little change in heart rate might result from an atrioventricular (atrioventricular [AV])
nodal junctional rhythm, and the hemodynamics in this case might be further compromised by the sympathetic discharge associated with an isorhythmic AV dissociation. Sometimes, merely reducing the depth of the inhalation anesthetic agent, or the substitution of another balanced anesthetic technique, may end the dysrhythmia and improve blood pressure.
nodal junctional rhythm, and the hemodynamics in this case might be further compromised by the sympathetic discharge associated with an isorhythmic AV dissociation. Sometimes, merely reducing the depth of the inhalation anesthetic agent, or the substitution of another balanced anesthetic technique, may end the dysrhythmia and improve blood pressure.
This chapter focuses on the origins, recognition, and treatment of the common atrial and ventricular perioperative dysrhythmias. His-Purkinje system (HPS) conduction blockade (“heart block”) will be discussed as well. The authors have assumed that that the reader has basic electrocardiographic knowledge.
What Are the Basic Facts About Bradydysrhythmias, and How Are They Managed?
▪ SINUS NODE DYSFUNCTION
The sinoatrial (SA) node and the atrium are intimately involved in the initiation of a cardiac cycle, and therefore any failure of these tissues can result in bradycardia. Conditions that lead to failure of heart beat initiation include:
Sinus node arrest (no spontaneous depolarization)
Sinus node exit block (SA node depolarizes but electric signal is not propagated within the region of the SA node)
Atrial tissue failure (the propagating depolarization fails to reach the AV node)
Often, without electrophysiologic study, differentiation of these conditions is difficult if not impossible. Abnormal electrolytes, preoperative β-blocker use, and many of the intraoperative drugs have the potential to aggravate bradycardia and bradycardia-dependent arrhythmias.4
Probably the most common bradycardia results from the slowing of the sinus node, as in our case summary. In the operating room, it can be caused by drugs, especially dexmedetomidine and vagotonic agents such as fentanyl, sufentanil, and remifentanil. In a retrospective analysis of 6,663 electronically recorded cases of neuraxial anesthesia, Lesser et al. found that a baseline heart rate <60 bpm and male gender were risk factors for severe (<40 bpm) bradycardia.5
▪ ATRIOVENTRICULAR NODAL BLOCK
Electric events that initiate cardiac contraction generally start in the sinus node, spread (or arborize) over the atrial
tissue, activate the AV node, and then traverse the HPS to activate the ventricles. In the presence of P waves, but without ventricular activation, AV nodal block is present. Atrioventricular block can be a temporary or permanent disturbance of AV impulse conduction due to anatomic or functional impairment of conduction. AV block is classified as first-, second-, or third-degree (complete) block. The level of AV block can also be defined as supra-, intra, or infra-Hisian block.
tissue, activate the AV node, and then traverse the HPS to activate the ventricles. In the presence of P waves, but without ventricular activation, AV nodal block is present. Atrioventricular block can be a temporary or permanent disturbance of AV impulse conduction due to anatomic or functional impairment of conduction. AV block is classified as first-, second-, or third-degree (complete) block. The level of AV block can also be defined as supra-, intra, or infra-Hisian block.
First Degree
First-degree AV block is a prolonged PR interval exceeding 200 ms. In general, this condition is benign, although it has been associated with significant bradycardia during spinal anesthesia.6 Progression to higher grades of AV block is rare in the general population,7 but it has been reported with spinal8 and general9 anesthesia. With a prolonged first-degree block exceeding 400 ms, apparent AV dyssynchrony can be present, and higher grade block can develop if the atrial rate increases with metabolic demand from exercise, trauma, anemia, as the HPS fails to conduct all of the atrial impulses to the ventricles. Treatment of first-degree heart block will depend upon symptomatology.
Second Degree
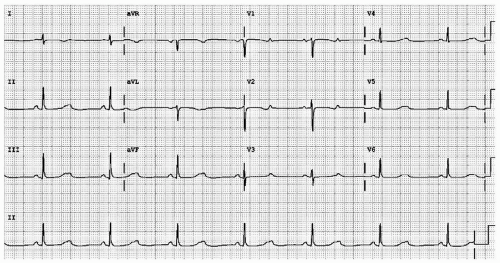
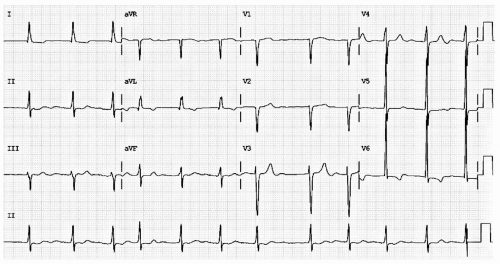
Second degree block represents disease along the HPS. In second degree AV block, some impulses are blocked. Classification depends upon the PR interval stability. In Mobitz I (also called Wenckebach) block, the PR interval progressively lengthens until the ventricles fail to activate. Assuming hemodynamic stability, Wenckebach block is treated with watchful waiting. In Mobitz II block, the PR interval is stable. Mobitz II represents more serious AV nodal/HPS disease and often requires pacemaker placement. Distinguishing Mobitz I from Mobitz II can be quite challenging, especially in the presence of a 2:1 block (see Fig. 18.3).
Third Degree
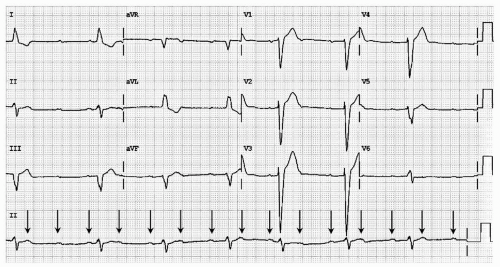
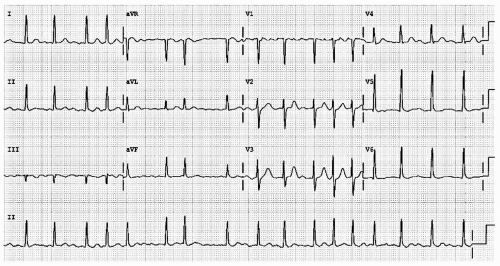
In third-degree AV block, complete failure of the HPS results in no atrial event being conducted to the ventricles. Ventricular systoles continue only in the presence of junctional or ventricular escape activity. The presence of P waves without a clear relation to the QRS complexes (rate and apparent PR interval) confirms the diagnosis of this problem (see Fig. 18.4).
Whether treatment (pharmacologic or pacing) becomes necessary depends upon the patient’s hemodynamic stability and medical condition. Pharmacologic therapy can include atropine, ephedrine, or epinephrine. Some practitioners might also consider glycopyrrolate, but it is indicated only for “vagally induced bradycardia.”10 Use of these chronotropic drugs can sometimes lead to uncontrolled sinus tachycardia.11
▪ PACING
In this setting, temporary cardiac pacing also can be considered. Pacing may be carried out through transcutaneous, transvenous, transthoracic (introduction of pacing wire[s] directly into the thorax), and transesophageal modalities. Transcutaneous and ventricular-only transvenous pacing, even if feasible, may exacerbate hemodynamic problems in patients with cardiomyopathy, as these pacing modalities do not preserve AV synchrony (i.e., they produce only ventricular or global myocardial activation). Although perioperative temporary pacing has been completely reviewed elsewhere,12 a few important aspects will be discussed.
Transvenous
Transvenous cardiac pacing provides the most reliable means of temporary pacing, albeit at the expense of the time required to arrange the equipment, establish central access, and determine the appropriate position of the ventricular catheter to provide ventricular capture. Flow-directed catheters and a right internal jugular approach afford the shortest insertion times.13 The reported incidence of successful capture in urgent situations without fluoroscopy ranges from 30% to 90%.14 Also, temporary pacing in the patient with a permanently placed pacemaker or implantable cardioverter-defibrillator (ICD) may be contraindicated without reprogramming, because the temporary pacing equipment can interfere with the permanent cardiac generator.
Most transvenous, flow-directed pacing catheters offer only ventricular pacing. The pulmonary artery AV pacing catheter, described by Zaidan in 1983,15 allows for AV sequential pacing through electrodes attached to the outside of the catheter, as well as routine pulmonary artery catheter functions. Combination of the two functions into one catheter eliminates the need for separate insertion of temporary transvenous pacing electrodes. However, several potential disadvantages exist with this catheter:
Varying success in initiating and maintaining capture15
External electrode displacement from the catheter16 and
Relatively high cost when compared with standard pulmonary artery catheters
The Paceport PAC provides ventricular pacing with a separate bipolar pacing lead (Chandler probe), which
allows more stable ventricular pacing as well as pulmonary artery catheter function.17 This catheter has been used for successful resuscitation in cardiac arrest during closed chest cardiac massage when transcutaneous and simple bipolar pacing had failed. A newer AV Paceport PAC adds another lumen to allow placement of another pacing lead for atrial pacing. The atrial wire can also be used to diagnose supraventricular tachydysrhythmias (supraventricular and ventricular tachycardia [SVT]) by atrial electrograms and to overdrive pace of atrial flutter (AFL) and reentrant SVT.18
allows more stable ventricular pacing as well as pulmonary artery catheter function.17 This catheter has been used for successful resuscitation in cardiac arrest during closed chest cardiac massage when transcutaneous and simple bipolar pacing had failed. A newer AV Paceport PAC adds another lumen to allow placement of another pacing lead for atrial pacing. The atrial wire can also be used to diagnose supraventricular tachydysrhythmias (supraventricular and ventricular tachycardia [SVT]) by atrial electrograms and to overdrive pace of atrial flutter (AFL) and reentrant SVT.18
Transcutaneous
Transcutaneous pacing, first described by Zoll,19 is readily available and can be rapidly implemented in emergency situations. Capture rate is variable, and the technique often causes pain in awake patients, but usually is tolerated until temporary transvenous pacing can be instituted. It may be effective even when endocardial pacing fails.20 It is now considered by many to be the method of choice for prophylactic and emergent applications.21
Transesophageal
Esophageal pacing is the newest technique available, and it has been shown to be quite reliable.22,23,24,25 Esophageal pacing is relatively noninvasive, well tolerated even in most awake patients, and it appears to be devoid of serious complications. It is contraindicated in the patient with atrial disease (e.g., atrial fibrillation (AF) or flutter), AV nodal disease, or any patient with a permanently implanted cardiac generator, because the electric output from the esophageal pacemaker can inhibit the output from the permanent device. This modality is useful for heart rate support of cardiac output, overdrive suppression of reentrant SVT, and for diagnostic atrial electrograms. Ventricular capture must be excluded before attempts are made at rapid atrial pacing for overdrive suppression to prevent potential VT or VF. Some surgical positions (e.g., prone) can increase the chance of unintentional ventricular capture, and esophageal atrial pacing should be followed very carefully.26 Typically, the pacing stimulus is delivered using a modified esophageal stethoscope, with the distal end of esophageal stethoscope inserted to a depth of 30 to 40 cm from the teeth. Capture should be confirmed using the peripheral pulse (i.e., from the pulse oximeter plethysmogram or an invasive hemodynamic monitor), because the pacing stimulus often is large relative to the QRS and frequently fools the electrocardiographic counting algorithm on the monitor. Atrial capture is obtained in virtually all patients using an indicated output of 8 to 20 mA; the output should be set to two to three times the threshold for capture. Thresholds are not influenced by weight, age, atrial size, or previous cardiac surgery.25 Because there is no sensing element involved, esophageal pacing is AOO <mode pacing. Transesophageal ventricular pacing is generally unreliable, yet the optimal site appears to be 2 to 4 cm distal to the atrial site.27 The esophageal stethoscope can also be used (with a special adapter) to record the intraatrial electrogram. Problems with esophageal pacing include:
The necessity for special generators that provide an output of 20 to 30 mA, with pulse width of 10 to 20 ms (typical temporary generators have a maximum output of 20 mA with pulse width duration of 1 to 2 ms)
The ability to pace only the left atrium and not the left ventricle, which can be a significant problem in emergency situations23
Phrenic nerve stimulation with significant diaphragmatic movement24 and
Induction of ventricular tachydysrhythmias during rapid atrial pacing has been noted. No long-term complications with this modality have been described, and no significant esophageal trauma has been reported despite long-term therapy of up to 60 hours.28
How Are Supraventricular Tachydysrhythmias Detected and Managed?
▪ ATRIAL PREMATURE COMPLEX
An atrial premature complex (APC) results from inappropriate early depolarization of atrial tissue, not under control of the sinus node, which might cause some form of P wave to be inscribed on the surface ECG (see Fig. 18.5). Subsequent HPS activation results in ventricular depolarization, inscribing a QRS complex often identical to that of the normal sinus beat. APCs frequently “reset” the sinus node timing, so the interval from the APC to the next true P wave might be less than fully compensatory.29 Frequently, the P wave from the APC remains “hidden” in the prior T wave. If a P wave is present, it usually differs in morphology from the normal sinus P wave, because it originates from a site different from the SA node. Because the distance from this aberrant atrial focus might be different from that of the SA node to the AV node, the PR interval also might be different from that of a sinus event. Although these morphologic features can help differentiate APCs from premature ventricular complexes (PVCs), none of these features is absolutely reliable. For instance, as noted in the preceding text, the aberrant P wave may fall upon the preceding T wave and becomes difficult to identify. The post-extrasystolic pause may appear fully compensated if there is a delay in the sinus node discharge of the following beat. When aberrant ventricular conduction occurs, the QRS complex may appear widened.
If the HPS is, in fact, redepolarized before complete repolarization of the conduction system or ventricular tissue, a bizarre, wide complex QRS can be inscribed on the surface ECG. Most commonly, this QRS will appear in a right bundle branch pattern—this event is termed the Ashman Phenomenon.30
A few simple criteria often help distinguish a wide complex QRS inscribed by an aberrantly conducted APC from a PVC. Generally, the initial deflection of an aberrantly conducted QRS is identical in direction to the sinus-induced QRS, and its configuration is similar to the right bundle branch block (RBBB) pattern with a duration <0.14 seconds. On the other hand, the QRS inscribed by a PVC shows an initial deflection to be opposite to that of sinus rhythm QRS, and its configuration is different from the RBBB pattern with a duration >0.14 seconds.
Hemodynamic Significance
In most cases, APCs are completely asymptomatic. When they occur frequently in the conscious patient, they may cause palpitations or an unpleasant feeling of irregular heart beats. APCs very early in the hyperexcitable phase of the cardiac cycle may precipitate tachyarrhythmias, in particular AF.31
Prevalence
Occasional APCs are very common, even in patients without underlying heart disease. In normal subjects, stress, physical exhaustion, heavy smoking, alcohol, and caffeine may induce APCs. The frequency of APCs increases with increasing age and in the presence of structural heart disease. The incidence of APCs is higher in patients with diseases of the mitral valve such as mitral stenosis and mitral valve prolapse, ischemic heart disease, and congestive heart failure. Noncardiac medical conditions associated with APCs include acute and chronic pulmonary diseases, chronic renal failure, and metabolic abnormalities.
Management
In asymptomatic cases, no treatment is required for occasional APCs. For patients with frequent symptomatic APCs, management begins with simple reassurance, along with identification and avoidance of precipitating factors such as stress or excess caffeine. If these measures fail to alleviate symptoms, drug therapy can be started with β-blockers,32 which may also help prevent APCs from triggering other more serious tachyarrhythmias such as AF.
▪ PAROXYSMAL REENTRANT SUPRAVENTRICULAR TACHYCARDIA
Paroxysmal reentrant supraventricular tachycardias are characterized by abrupt onset and regularity. The pathophysiology of these arrhythmias involves two tissues that have different conduction velocities and refractory periods (slow and fast pathways). The impulse travels down one
pathway while the second is in the refractory period, then travels up the second, thereby perpetuating the arrhythmia. The most common paroxysmal reentrant supraventricular tachycardia is AV nodal reentrant tachycardia, which involves reentry within the AV node. AV nodal reentrant tachycardia usually demonstrates a regular, narrow complex tachycardia of 160 to 180 bpm. It is generally benign unless structural heart disease is present. Patients typically present with palpitations and shortness of breath.
pathway while the second is in the refractory period, then travels up the second, thereby perpetuating the arrhythmia. The most common paroxysmal reentrant supraventricular tachycardia is AV nodal reentrant tachycardia, which involves reentry within the AV node. AV nodal reentrant tachycardia usually demonstrates a regular, narrow complex tachycardia of 160 to 180 bpm. It is generally benign unless structural heart disease is present. Patients typically present with palpitations and shortness of breath.
Management
Vagal maneuvers, such as Valsalva maneuver or carotid massage (after ensuring there is no carotid bruit) usually terminate the tachycardia. Adenosine can be used with good success rates. AV nodal blocking agents, such as β-blockers and calcium channel blockers, often are effective in terminating and preventing the recurrence of AV nodal reentrant tachycardia. Patients with refractory AV nodal reentrant tachycardia or those who do wish to take medication can undergo catheter ablation.
▪ ATRIAL FIBRILLATION AND ATRIAL FLUTTER
AF is the most common sustained atrial arrhythmia encountered in anesthesia practice.33 AF represents loss of the sinus node as the primary cardiac pacemaker, being replaced by totally disorganized atrial activity with rapid fibrillatory waves with varying morphology and an irregularly irregular ventricular rhythm on the ECG (see Fig. 18.6). The QRS complex is usually narrow but may be wide in cases of coexisting bundle branch blocks or aberrant conduction. With QRS complexes of varying amplitudes and total irregularity of the arterial pulse, this rhythm has often been classically referred to as delirium cordis. This arrhythmia has important clinical implications because patients with AF have increased risk for morbidity and mortality. AF can often lead to symptoms impairing patients’ functional status and quality of life.
AFL is sometimes seen with AF. It is characterized by more regular atrial activity, with a saw-tooth pattern on ECG, and the ventricular response is generally regular because of a 2:1, 3:1, or greater atrial-to-ventricular conduction pattern (see Fig. 18.7). Sustained AFL is less common than AF, and AFL generally degenerates to AF. The evaluation and management of AFL is identical to that of AF and will be discussed in the subsequent text.
Epidemiology
The overall prevalence of AF is estimated to be one percent of the total population. The prevalence increases with advancing age—from 0.1% for people younger than 55 years to 9% in those older than 80 years34 Prevalence is higher
in men than in women, and higher in whites than African Americans. The risk of AF increases with cardiovascular diseases such as hypertension, ischemic heart disease, valvular heart disease, and sick sinus syndrome.35
in men than in women, and higher in whites than African Americans. The risk of AF increases with cardiovascular diseases such as hypertension, ischemic heart disease, valvular heart disease, and sick sinus syndrome.35
Classification
AF has been classified based upon its morphology and appearance on ECG. The baseline undulations may be clearly distinct and visible (coarse AF), intermediate (medium AF), or barely discernable (fine AF). To the extreme, there may be no perceptible undulation of the baseline. There is no consistent correlation of these different types of AF with either the severity of AF or its associated underlying cardiac conditions. Currently, the American College of Cardiology/American Heart Association/European Society of Cardiology (ACC/AHA/ESC) classifies AF into four types as follows:36
PAROXYSMAL: Sudden AF that spontaneously reverts; that is, the abnormal rhythm self-terminates in < 7 days (usually <24 hours) and may be recurrent, defined by two or more episodes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree