Mechanism of action of local anesthetics (LA) at the sodium channel with subsequent blocking of neural transmission. Normal neural transmission is dependent on sodium (Na+) influx through Na+ channels with potassium (K+) outflow restoring resting membrane potential. This pathway is blocked by LA acting at these channels. LA enters the cell in the non-ionized form and is in equilibrium with hydrogen (H+) ions with the ionized form active at the sodium channel.
The channels become activated after chemical, mechanical, or electrical stimuli (i.e. pain impulse). As sodium ions enter into the cell an action potential is generated that is conducted as a nerve impulse (Figure 3.2). LAs bind and inhibit the sodium channels from inside the cell via reversible ionic interactions with the alpha subunit of the sodium channel. It is the ionized fraction that binds with higher affinity and dissociates from the channel subunit at a slower rate. This prevents cell membrane depolarization by blocking sodium ions from entering into the cell and preventing the channel from changing its conformation. The resulting interruption of signal conduction blocks pain transmission and can also lead to motor blockade.
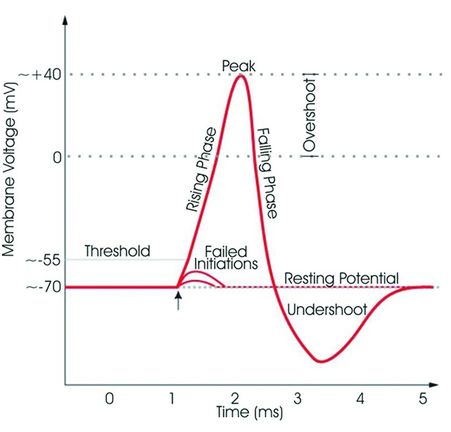
Neural action potential. Sodium channels open with subsequent sodium ion influx once the membrane potential voltage reaches –55 mV (threshold). Once the voltage of +40 mV is reached, potassium channels open with the outflow of potassium ions restoring the membrane potential. (Copyright © Synaptitude 2005. Permission is granted to copy, distribute and/or modify this document under the terms of the GNU Free Documentation License, Version 1.3 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. A copy of the license is included in the section entitled “GNU Free Documentation License.”)
LA action is not limited to peripheral nerve cells. They also affect the myocardium and the central nervous system (CNS). For example, lidocaine has antiarrhythmic properties. At low levels, lidocaine is an anticonvulsant (Mazoit and Dalens, 2004). In toxic doses, however, LAs can lead to adverse CNS events and even cardiovascular collapse.
Sensitivity to blockade
The physical characteristics of peripheral nerve fibers determine sensitivity to blockade by LAs. Sensitivity to LAs is inversely proportional to the increasing diameter, degree of myelination, and conduction velocity. Sensitivity of the nerve to LA blockade is decreased with increasing nerve diameter and increased conduction velocity. Motor nerves are the least sensitive to the effects of LAs as they are large in diameter, have higher conduction velocities, and are myelinated. Sensory fibers are smaller than motor fibers, with some myelinated and some unmyelinated, and thus more sensitive to LAs than motor nerves. Sympathetic, autonomic fibers are small and unmyelinated, rendering them first to be blocked by LAs during neuraxial anesthesia.
Local anesthetic agents
LAs are classified based on their chemical structure. Each LA has an intermediate chain linkage that binds an aromatic group to a tertiary amine. This linkage exists as either an amino-ester or amino-amide bond (Figure 3.3). Ester LAs include chloroprocaine, cocaine, procaine, and tetracaine. Amide LAs include lidocaine, mepivacaine, bupivacaine, ropivacaine, and levobupivacaine.
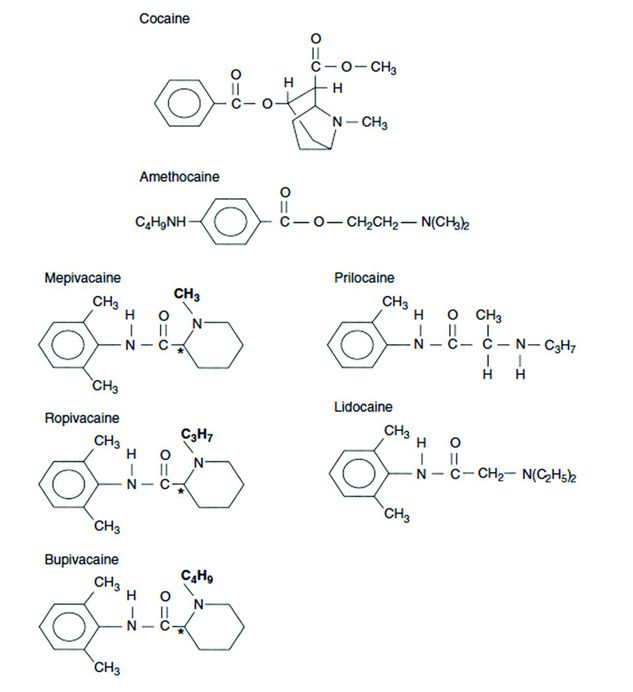
Chemical structure of different local anesthetics. Cocaine and amethocaine (tetracaine) are esters. The remainder are amides which have an NH functional group in the intermediate chain linkage.
Amide LAs are longer acting as a result of protein binding and hepatic metabolism, and are typically more suitable for regional techniques.
Potency, onset of action, and duration are pharmacologic principles to consider with LA administration (Table 3.1). Potency refers to the affinity of the LA for the lipid cell membrane of the neuron. More lipid soluble agents have higher potency and higher affinity for binding sodium channels.
| Potency | Refers to a LA’s affinity for sodium-channel binding Higher lipid solubility = increased potency Affected by nerve diameter and myelination |
| Onset of action | Related to the pKa of a LA Decreased pKa = increased non-ionized fraction of LA present and more rapid onset Sodium bicarbonate can be added to commercially prepared acidic LA solutions to quicken onset |
| Duration of action | Increased lipid solubility = increased duration Vasoconstriction = increased duration Epinephrine can be added to increase duration |
Onset of action relates to the pKa of a drug, which is the pH at which the non-ionized form and ionized form are in equal concentration. The ratio of ionized to non-ionized is given by the Henderson–Hasselbalch equation: pKa = pH + log[B]/[BH+]. The non-ionized form passes through the membrane into the neuron. At a low pKa, less of the ionized fraction and more of the non-ionized fraction is present, allowing for more rapid onset. The concentration and volume also affect the onset. While 3% chloroprocaine has a high pKa, it has a rapid onset secondary to a high concentration (Berde and Strichartz, 2010). The high concentration allows for quick diffusion into the cell. In addition, nerve fiber diameter and myelination affect drug potency.
The duration of action of LAs is also related to lipid solubility. The higher the lipid solubility the longer the duration of action, because of slower systemic absorption from the injection site. More lipophilic LAs have a higher degree of tissue binding, which inhibits systemic absorption. The intrinsic vasoconstrictive properties of ropivacaine also prolong its duration of action.
Additives are sometimes used to speed onset and increase duration of blockade. First, LAs are weak bases, but are prepared as acidic solutions to maintain stability. The addition of sodium bicarbonate at the time of the block shortens the onset by increasing the pH and thus increasing the lipophilic portion of the drug (B.HCl + HCO3−⇌B+H2CO3+Cl−). The addition of sodium bicarbonate to a LA may shorten the onset of peripheral and epidural nerve blocks by 3–5 minutes (Stoelting and Hillier, 2006).
Another commonly used additive is epinephrine. Epinephrine can increase duration of blockade of certain LAs secondary to vasoconstriction. The vasoconstriction decreases systemic absorption; it can especially impact neonates and children who have higher cardiac output. Some practitioners caution against using epinephrine with epidural anesthesia in children given the potential to cause spinal cord ischemia (Berde and Greco, 2012).
Protein binding
Amide LAs bind to serum proteins, mainly alpha-1 acid glycoprotein (AAG). The degree of protein binding varies for the different LAs, with lidocaine having 65% protein binding in adults and bupivacaine and ropivacaine having 95% protein binding (Mazoit and Dalens, 2004). Also, the amount of AAG in the serum varies between children less than 1 year old and that in older children or adults. The concentration increases over the first year from 0.2–0.3g/L at birth to 0.7–1.0g/l at 1 year, which is same concentration as in adults (Mazoit and Dalens, 2004). Thus, in children less than 1 year of age, more drug exists in the unbound free form, which increases the potential for systemic toxicity. It is absolutely necessary to understand that infants have decreased LA protein binding secondary to lower AAG levels. The traditional concept is that lower concentrations of AAG result in increased free drug (LA) availability and, hence, increased risk of toxicity (Polaner et al., 2010). However, as there is a dynamic interaction between free LA in equilibrium with the fraction bound to proteins and to the tissues, the clinical significance of lower AAG concentrations is unclear (Lönnqvist, 2012). As part of the acute phase response, AAG concentrations have been shown to increase after stress and surgery in infants, further reducing the effects of protein binding as a factor in LAST. Caution is advised when directly translating the maximum allowable dose of LAs adults to clinical use in infants It is recommended that doses should be reduced in very young infants (Dalens, 2010).
In addition to AAG binding, LAs bind to human serum albumin. This interaction holds less clinical significance as LA affinity is much lower for albumin than AAG, especially at normal clinical levels. Albumin can become a clinically significant buffer when AAG sites become saturated at toxic doses (Mazoit and Dalens, 2004). In addition to serum proteins, amide LAs also bind to red blood cells. Twenty to thirty percent of the drug in circulation is bound to erythrocytes. Since erythrocyte-binding sites are not saturated in clinical situations, this binding can also act as a buffer when toxic levels arise (Mazoit and Dalens, 2004). Red blood count binding might be more significant in neonates as they naturally have polycythemia and macrocythemia, allowing for more LA binding to help prevent toxicity (Mazoit and Dalens, 2004).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








