Characteristic
Age
APACHE IIa
SOFAb score
Number of comorbidities
Inpatient floor stay prior to ICUc admit?
IL-6d
Points
≥50 = 1,≥75 = 2
≥15 = 1
≥20 = 2
≥28 = 3
≥6 = 1
≥10 = 2
≥2 = 1
Yes = 1
≥400 = 1
The use of structured protocols and algorithms to manage nutrition support in the ICU is backed by high-level evidence and is associated with improved patient outcomes [17, 18]. An example of such a feeding algorithm is found in Fig. 18.1. Moreover, nutrition therapy is optimized by the use of multidisciplinary nutrition support teams, dietitians [19], and nurse-driven protocols [10].
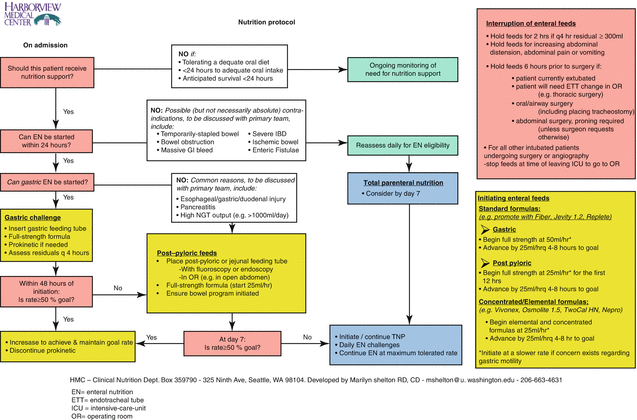

Fig. 18.1
Illustrative example of an intensive-care-unit nutrition protocol. EN enteral nutrition, ETT endotracheal tube, ICU intensive-care-unit, OR operating room
18.4 Calculating Energy Requirements
Depending on the characteristics of the patient and their illness severity, resting energy requirements can vary substantially between individuals. Current tools to capture this variation and characterize “nutrition status” are limited [20]. This is mainly because traditional biochemical indices for nutrition status (albumin, prealbumin, and transferrin) are not valid in the ICU setting. These markers, rather than reflecting calorie intake or underlying “nutritional reserve,” more likely reflect the presence of vascular permeability and hepatic synthetic reprioritization during acute illness [10]. Moreover, other methods like calorimetry, nitrogen balance, and anthropometry are labor intensive and imprecise, and have not been associated with improved outcomes [20]. Common formulas such as the Harris-Benedict equation can help assess calorie needs, but these formulas were not designed and validated in a critical-care population. Therefore, determination of calorie targets in the critically ill is a blend of art and science, where the provider must utilize existing imperfect methods combined with their clinical judgment and consultation with nutrition support teams.
With these caveats in mind, a simple (albeit imprecise) method is to use weight-based estimates of calorie requirements. In the ICU, the recommended range for calorie intake is 20–40 kcal/kg/day, with 1.5–2 g/kg/day of protein. Obesity requires a special consideration: for BMI 30–50, the recommended calorie intake is 11–14 kcal/kg/day actual body weight, while for BMI > 50, the recommended intake is 22–25 kcal/kg/day ideal body weight [10]. These estimates must be adjusted upward for “high-risk” patients (those who are elderly, have severe injury, or significant traumatic brain injury [TBI], are previously malnourished, or have a NUTRIC score ≥5).
18.5 Enteral Nutrition (EN)
EN therapy is the preferred primary nutrition therapy and has several advantages over parenteral nutrition (PN) therapy. Multiple trials and meta-analyses have demonstrated that relative to PN, EN reduces overall complications, infections, cost, and LOS [1, 10].
However, known risks of EN include gut ischemia, vomiting, and aspiration pneumonia. The critical-care surgeon should always be aware of the potential for gut ischemia and necrosis with EN therapy and must consider this in the setting of distension, diarrhea, or acidosis. Basic essential practices that lower the risks for aspiration pneumonia include head of bed elevation, oropharyngeal decontamination, and continuous rather than bolus feeding. Monitoring gastric residual volumes may not be clinically meaningful; about 20 % of patients with “low” residual volumes (<150 cc) still have aspiration events and those with high residual volumes (up to 300 cc) do not appear to have any higher aspiration risk [21–23].
Contraindications to EN therapy are few and include gastrointestinal discontinuity and hemodynamic instability. EN in a patient with persistent hypotension and/or vasopressor use increases the risk for bowel ischemia in the setting of an already compromised intestinal microcirculation. Relative contraindications for EN include irritable bowel disease (IBD) acute flares, enteric fistulae, and gastrointestinal bleeding.
Diarrhea is a common problem facing the ICU patient on EN, and should not automatically lead to cessation of feeds. Generally, if the patient has >300 mL/day or >4 loose stools/day, a Clostridium difficile toxin assay should be sent, and other infectious sources should be considered. Once infection is ruled out, adding 10–20 g soluble fiber and/or switching to elemental formula may help decrease diarrhea [17]. If the problem persists, consider use of supplemental PN (see next section).
There are some special circumstances related to feeding route. Postpyloric feeding tubes should be considered if the patient has (a) signs of gastric intolerance, (b) high aspiration risk, or (c) injury to the esophagus, stomach, or duodenum. Placement of postpyloric tubes in pancreatitis has recently been called into question and is no longer clinically indicated [10]. Surgical feeding tubes are associated with a decreased risk for aspiration relative to nasogastric or orogastric tubes [23] and should be considered in patients anticipated to require EN beyond 14 days.
Recommendations continue to evolve regarding timing for EN calorie delivery. Early enteral nutrition, given in the first 24–48 h of ICU admission, has consistently been associated with reduced infections and mortality in several observational cohorts [10, 24]. However, most of these studies are subject to residual confounding by indication (patients who are less ill tend to be successfully fed earlier). Adequately powered randomized trials are needed to more definitively address the issue of timing.
Optimal calorie goals are an ongoing controversy in nutrition science. Recent literature indicates that trophic feeds (“trickle feeds” at a rate of 10–20 ml/h) may be equivalent to full goal calorie feeds [25], and in some cases, achieving 100 % of the daily calorie goal may actually increase infection rates, days of mechanical ventilation, and LOS, when compared to trophic feeding [26, 27]. Practically speaking, trophic feeding or hypocaloric feeding is a reasonable strategy for low-risk patients in the first 7 days of ICU stay, but high-risk patients (e.g., a NUTRIC score ≥ 5) should probably still reach ≥80 % goal calorie and protein intake as quickly as possible [10]. Strategies to minimize “nil-per-os” periods should be employed, including allowing some intubated patients to continue EN up until the time of transfer to the operating room, or continued through select operations [9].
18.6 Parenteral Nutrition (PN)
PN should be initiated only after a trial of all enteral routes. EN is generally preferred over PN because several randomized controlled studies have demonstrated that PN decreases immune function, increases the risk of infections, and prolongs hospital LOS, when compared to EN [10, 14]. PN use has generally declined over the past decade due to a growing recognition of its associated morbidity [28]. Some subpopulations of patients may still benefit from PN therapy, but these patients are often the most metabolically and medically complex. For this reason, a multidisciplinary nutrition support team with dietitian support provides essential assistance in these nuanced and difficult treatment decisions [19, 29].
PN should be considered in patients with absolute or relative contraindications for EN, or for those who are EN “intolerant.” For “low-risk” patients (NUTRIC score <5) who are unable to achieve goal calories via an enteral route, trophic feeding should continue without attempting to “supplement” calorie intake with PN. In the “low-risk” enteral-intolerant patient, good quality evidence indicates that starting supplemental PN prior to hospital day 7 is associated with prolonged length of stay, increased days of mechanical ventilation, and increased infections [30, 31]. However, starting PN before day 7 may benefit “high-risk” (NUTRIC score >5/preexisting malnutrition) enteral-intolerant patients. PN should also be considered for patients who continue to fail in enteral therapy beyond 7 days [32].
For those patients who have a contraindication to any early enteral intake (including tropic feeding), PN therapy may have some benefit. In this population, PN therapy appears to cause no additional morbidity or infections, and may even improve lean body mass and total vent free days [33]. These findings are particularly relevant for surgical patients, since many perioperative patients have contraindications to enteral intake. A summary of an evidence-based approach to EN and PN is given in Table 18.2.
Table 18.2
Evidence-based priorities for enteral nutrition (EN) and parenteral nutrition (PN) in the first 7 days of intensive care unit stay
Start EN within 24–48 h. Titrate to goal calorie intake |
If unable to achieve goal enteral calorie intake: |
In “low risk”a patient: maintain trophic feeds up to 7 days without supplemental PN |
In “high risk”a patient: consider starting supplemental PN within 48 h |
If contraindicationb to all enteral intake anticipated beyond 72 h, start early PN |








