Embolism
Thrombosis
1. Atrial fibrillation
1. Atherosclerotic occlusion
2. Mural thrombus
2. Thrombosed aneurysm
3. LV aneurysm
3. Bypass or stent graft occlusion
4. Paradoxical
4. Hypercoagulable states
5. Endocarditis
5. Dissection
6. Atrial myxoma
6. Vasospasm
7. Aortic/large vessel atheroma
7. Iatrogenic
Emboli can arise from the heart (80–90 %) or diseased proximal arteries (10–20 %). Common cardiac causes include atrial thrombus with atrial fibrillation and mural thrombus in acute coronary syndrome and left ventricular dysfunction, to septic emboli in infective endocarditis, thrombus with prosthetic valves and myxoma much less frequently. Also, atherosclerotic plaque or mural thrombus can embolize from a diseased proximal artery.
Acute thrombosis most often occurs at a site of atherosclerotic plaque in peripheral arteries either the plaque hemorrhages, exposing the hypercoaguable subendothelial collagen, which leads to thrombus formation or the plaque causes a critical stenosis, which reduces the blood flow, promoting thrombus formation. Vein bypass grafts can thrombose in the context of an anastomotic stenosis, a kink in the graft or if there is a retained valve cusp, all of which result in a flow disturbance or low-flow state. Thrombosed vein grafts are often preceeded by clinical symptoms or arterial compromise (e.g. claudication) prior to thrombosing. A prosthetic graft can thrombose at any point often without any prodrome or warning signs. Large vessel aneurysms – most commonly aortic, popliteal, and femoral – cause turbulent flow, which is prothrombotic. Some prothrombotic states can result in arterial thrombosis, for example, antiphospholipid syndrome, heparin-induced thrombocytopenia, or malignancy, as can systemic low flow (hypotension) in an unwell patient. Rare causes of an acute ischemic limb include compartment syndrome, dissection, external compression, cystic adventitial disease, and popliteal entrapment.
11.3 Diagnosis
The history and examination in the diagnosis of acute limb ischemia revolves around the “six P’s” – pulselessness, pain, pallor, paralysis, paresthesia, and poikilothermia. Patients with embolic limb ischemia often report the sudden onset of limb pain and paresthesia (often able to pinpoint their exact action/movements at the time of onset), followed shortly by pallor and varying degree of paralysis depending on the arterial bed involved.
Unlike lower limb ischemia, upper limb ischemia is almost exclusively due to embolic causes. Atherosclerotic occlusive disease is extremely unusual in the upper limb with numerous collateral pathways providing adequate flow to ensure ischemia is rarely limb threatening, and thus treatment can be approached in a less urgent fashion.
The contralateral limb and abdomen should always be examined carefully as they provide vital clues as to the underlying etiology. One must strongly suspect an embolic cause, particularly if the asymptomatic limb has a full complement of palpable pulses. A prominent pulse in the abdomen or in the popliteal fossa of the contralateral leg suggests an aortic or popliteal aneurysm respectively, particularly if a mass can be felt in the popliteal fossa of the affected leg (Fig. 11.1). Lack of pulses in the contralateral extremity may be a sign of chronic underlying stenotic disease as arterial disease often affects both limbs in a mirror pattern.
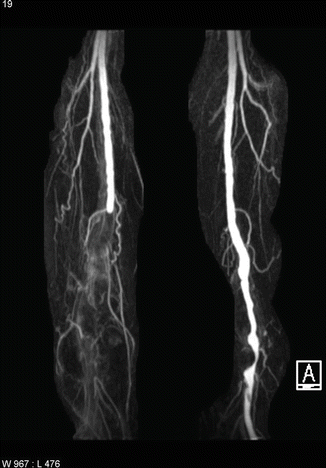

Fig. 11.1
MRA showing occluded popliteal aneurysm on the right and patent but aneurysmal popliteal artery on the left
In patients with thrombotic occlusion, there is often a history of low-grade ischemia, most often presenting as intermittent claudication, suggesting an underlying impairment of normal arterial flow prior to the acute presentation.
Other nonatherosclerotic and nonembolic causes should be suspected in those patients who are relatively young, without the typical risk factor profile or with another diagnosis that predisposes them to a thrombotic condition (e.g., thrombocytosis, polycythemia rubra vera, malignancy).
On examination, the ischemic limb is initially cool, pale, with delayed capillary refill and with or without changes associated with chronic ischemia (atrophic skin, loss of hair, and nail thickening). After 6–12 h, the limb can turn red due to vasodilation, after which it becomes mottled and blanches, and finally mottled with fixed skin staining. Pulses must be examined bilaterally from the femoral to the popliteal, dorsalis pedis, and posterior tibial. If pulses are absent, a handheld continuous Doppler should be used to assess for an audible signal, suggesting residual blood flow. Finally, it is important to examine for motor-sensory deficits. Based on the examination findings, the limb can be classified in terms of viability and urgency of revascularization (see table below).
Description | Capp. refill | Muscle paralysis | Sensory loss | Arterial Doppler signals | Venous Doppler signals | |
|---|---|---|---|---|---|---|
I – Viable | Not immediately threatened | Intact | None | None | Audible | Audible |
IIa – threatened | Salvageable if treated promptly | Slow | None | Partial | Inaudible | Audible |
IIb – threatened | Salvageable if treated immediately | Slow/absent | Partial | Partial/complete | Inaudible | Audible |
III – irreversible | Not salvageable | Absent. Fixed staining | Complete. Tense compartment | Complete | Inaudible | Inaudible |
Critical limb ischemia occurs when a decrease in perfusion threatens the viability of the limb, manifesting in rest pain, tissue loss, and/or gangrene.
11.4 Investigations
Urgent blood tests should include a full blood count, renal function, and coagulation profile to assist the choice of imaging modality and preparation for surgery. An electrocardiogram (ECG) should also be done routinely on admission to check for cardiac arrhythmias and excluded recent/current coronary ischemia. A chest x-ray may be useful in identifying undiagnosed congestive cardiac failure. The most appropriate imaging modality will depend upon what is available at the time, institutional factors, and patient factors – renal function, body habitus, and implanted devices, to name a few.
It is completely appropriate to proceed straight to surgical intervention without any imaging in some patient cohorts, namely, those with a good history, risk factors, and physical examination supportive of sudden embolic event. Any suggestion that there may be a proximal inflow stenosis or disease should ideally be investigated further with an imaging modality that is most appropriate and available at the time of presentation to the institution.
11.4.1 Arterial Duplex Ultrasound
Duplex ultrasound combines B-mode with color Doppler to assess both the arterial anatomy and blood flow characteristics. Ultrasound is noninvasive, inexpensive, and has good sensitivity and specificity in identifying lesions; however, it is highly operator dependent. Furthermore, it can be difficult to assess iliac vessels behind bowel gas.
11.4.2 Computed Tomographic Angiography
With recent technological improvements (multidetector, thinner slices), computed tomographic angiography (CTA) produces high spatial resolution images of the peripheral arterial tree. It can also assess lesions that have been previously stented. Arterial calcification, however, causes beam-hardening artifact, making interpretation of some lesions difficult, especially in small caliber vessels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







