Fig. 41.1
(a) The common surface landmarks of the vertebral column. (b) The common surface landmarks of the vertebral column (With permission from Danilo Jankovic)
The spinous processes are angled caudally at the thoracic levels. However, they are nearly horizontal at cervical and lumbar levels (Fig. 41.2a–c) [8–10]. As a result, cranial angulation of the needle is required when performing a thoracic epidural, while needle insertion perpendicular to the skin usually suffices in the lumbar spine.
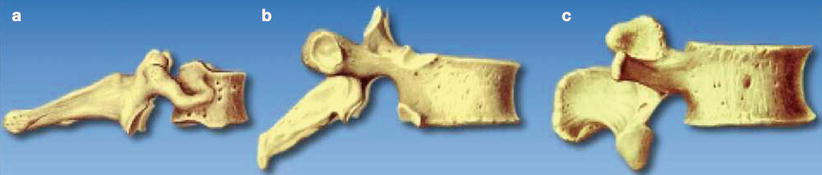

Fig. 41.2
Cervical, thoracic, and lumbar spinous processes (With permission from Danilo Jankovic). (a) C7 cervical vertebra (vertebra prominens, nuchal tubercle). (b) T8 thoracic vertebra. (c) L3 lumbar vertebra
The kyphotic thoracic curve and the lordotic lumbar spine determine the spread of subarachnoid local anesthetic in a supine adult. An injection of hyperbaric local anesthetic placed at the height of the lumbar lordosis will spread both caudad and cephalad to a variable extent (Fig. 41.3a, b). The cephalad extent of this spread is limited by the pooling of the solution at the mid-thoracic concavity [11].
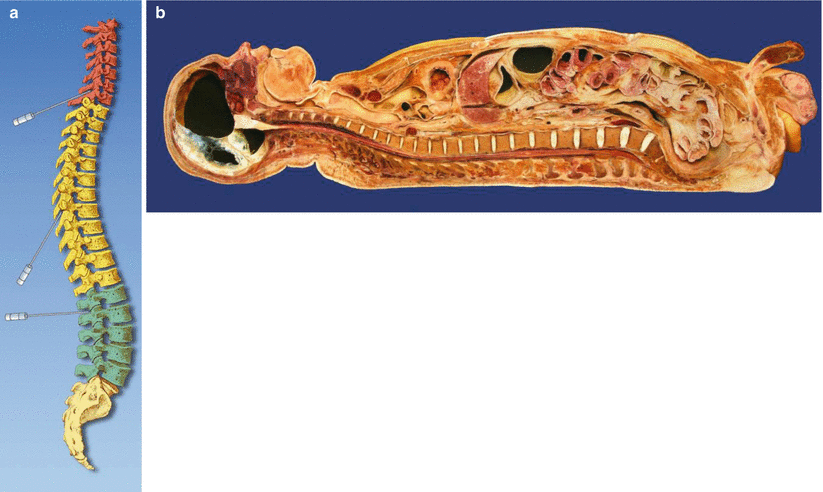

Fig. 41.3
(a) Anatomy: cervical, thoracic, and lumbar spine (With permission from Danilo Jankovic). (b) Paramedian sagittal section. Cervical, thoracic, and lumbar spine (With permission from Danilo Jankovic)
The three meningeal layers cover the spinal cord and protect it. The dura mater (Fig. 41.4) is the outermost meningeal layer and extends from the base of the skull to the second sacral vertebra. It is thickest in the posterior midline and thinner in the lumbar area than the thoracic or cervical levels [12, 13]. The epidural space is superficial to this layer and is the target for epidural anesthesia. The subdural space lies deep to this layer but superficial to the arachnoid mater.
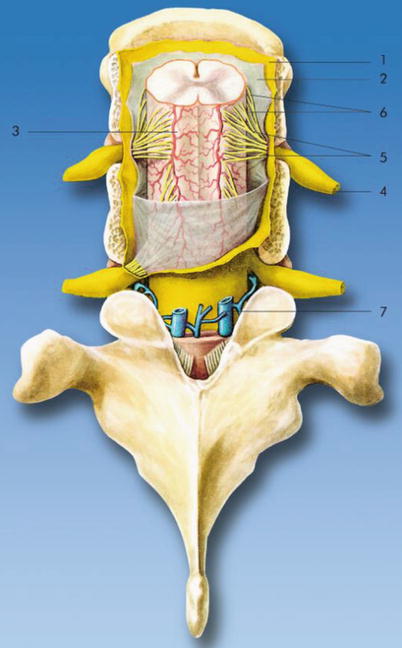

Fig. 41.4
Meninges. (1) Spinal dura mater, (2) arachnoid mater, (3) pia mater, (4) spinal nerve, (5) dorsal (posterior) nerve root, (6) ventral (anterior) nerve root, and (7) internal vertebral venous plexus (With permission from Danilo Jankovic)
The arachnoid mater lies deep to the dura mater and encloses the subarachnoid space and the cerebrospinal fluid (Fig. 41.4). Both spinal cord and spinal nerve roots are exposed to cerebrospinal fluid and thus are the sites of action of local anesthetic deposited in the subarachnoid space during a spinal anesthetic. Cysts in the subarachnoid space have been reported and may be a potential cause of inadequate spinal anesthesia [14, 15].
The pia mater is the innermost meningeal layer and covers the spinal cord (Fig. 41.4). The pia mater extends to the tip of the spinal cord and continues as the filum terminale, anchoring the spinal cord to the sacrum.
A needle inserted in the midline will traverse the skin, subcutaneous tissue, supraspinous ligament, interspinous ligament, ligamentum flavum, epidural space, dura mater, subdural space, and arachnoid mater to access the subarachnoid space (Fig. 41.5a, b). A paramedian insertion of the needle, on the other hand, bypasses the supraspinous and interspinous ligaments and can be useful in the older patient with degenerative spine disease and narrowed interspinous spaces.
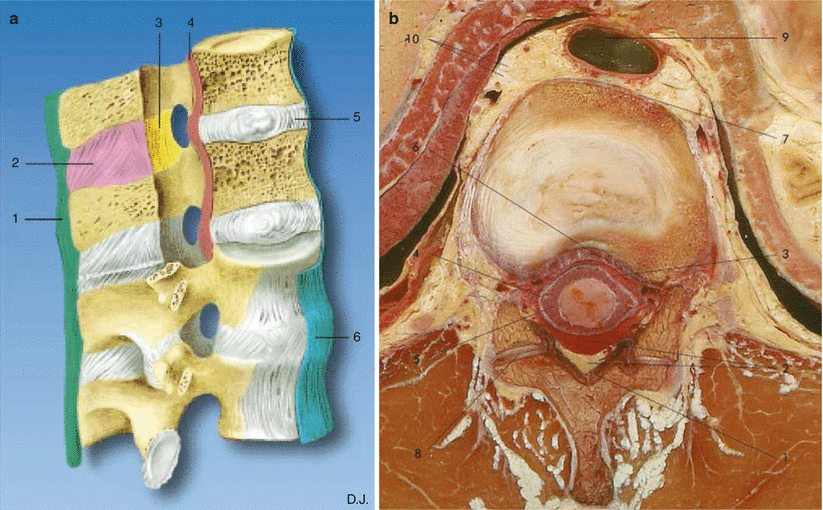

Fig. 41.5
(a) Sagittal section of the vertebral column illustrating the ligaments of the spinal cord. (1) Supraspinous ligament, (2) interspinous ligament, (3) ligamentum flavum, (4) posterior longitudinal ligament, (5) intervertebral disc, and (6) anterior longitudinal ligament (With permission from Danilo Jankovic). (b) Transverse dissection at the level of T9. (1) Ligamentum flavum, (2) posterior epidural space with fat, (3) anterior epidural space with veins, (4) spinal dura mater, (5) subarachnoid space with spinal cord, (6) posterior longitudinal ligament, (7) anterior longitudinal ligament, (8) zygapophysial joint, (9) aorta, and (10) sympathetic ganglion (With permission from Danilo Jankovic)
The length of the spinal cord varies with age. In the fetus, the spinal cord extends to the end of the vertebral column. However, by birth, the terminal end of the cord, the conus medullaris, extends only until L3 due to a faster growth of the vertebrae than the spinal cord. In the majority of adults, the conus ends at L1. Spinal anesthetics must therefore be performed at the L2–L3 intervertebral space or lower to avoid the risk of spinal cord injury [16]. The portion of the spinal cord that gives rise to the dorsal nerve root, ventral nerve root, and paired spinal nerves is called the spinal cord segment. The area of the skin supplied by a given cord segment and its spinal nerve is termed a dermatome. Qualitative assessment of sensory changes within the dermatomes allows rapid estimation of the spread of the local anesthetic within the subarachnoid or epidural space and is used to assess and document the extent of spinal or epidural anesthesia. Table 41.1 lists the most clinically relevant dermatomal levels and their corresponding surface landmarks.
Dermatomal level | Surface landmark |
|---|---|
C8 | Little finger |
T1–T2 | Inner arm |
T4 | Nipples |
T6 | Xiphoid |
T7 | Inferior border of scapula |
T10 | Umbilicus |
T12 | Inguinal ligament |
L2–L3 | Anterior thigh |
L3 | Patella |
L5 | Ankle |
S1 | Heel |
S2 | Back of the leg, back of the thigh, gluteal region |
S3–S5 | Perianal region |
The ligamentum flavum and interspinous ligaments are not continuous (Fig. 41.5a). While the interspinous ligament extends from the spinous process above to the spinous process below, the ligamentum flavum extends from the lamina superior to a given intervertebral space to the lamina inferior to it. Failure of the left- and the right-sided ligamentum flavum to fuse in the midline has been described and may result in difficulty in localizing epidural space using a midline “loss-of-resistance” technique [17].
The epidural space is not a continuous space, but segmented along its length (Fig. 41.6) [18]. Cryomicrotome sectioning and imaging has shown that the various compartments of the epidural space are segmented by areas where the dura is directly fused with the bone. The lateral epidural compartment is divided by intervening pedicles which are in contact with the dura, while the posterior epidural compartment is divided by dural contact with bone beneath the cephalad half of each lamina. The anterior epidural compartment is divided by the attachment of the posterior longitudinal ligament to the intervertebral disc at each level. These posterior, lateral, and anterior compartments of the epidural space may impede the movement of injectate and may explain the unpredictable epidural drug spread that is occasionally seen [19].
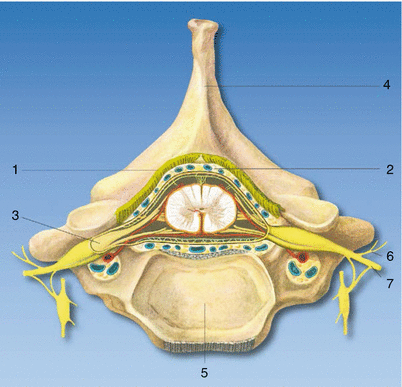
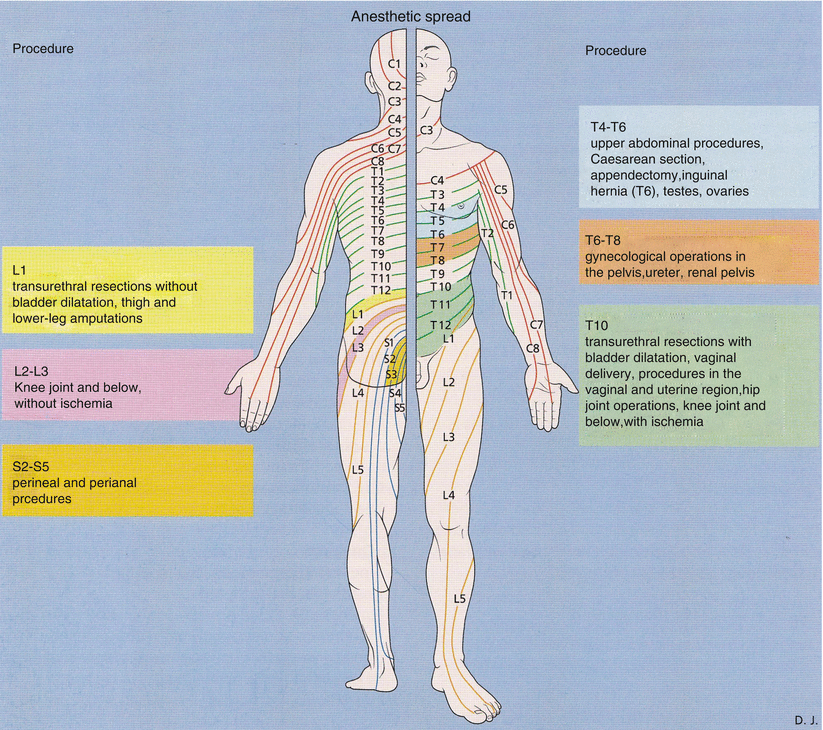

Fig. 41.6
Cross section of the epidural space. (1) Ligamentum flavum, (2) epidural space, (3) spinal ganglion, (4) spinous process, (5) body of vertebra, (6) dorsal branch of spinal nerve, and (7) ventral branch of spinal nerve (With permission from Danilo Jankovic)

Fig. 41.7
Location of the procedure and required sensory spread of local anesthetic (With permission from Danilo Jankovic)
Epidural fat in the epidural space (see also Fig. 41.5b) may play an important role in the pharmacokinetics of epidurally administered lipophilic drugs by acting as a reservoir. This may result in a delayed onset and a longer duration of action [20, 21]. A reduction in the epidural fat with age may partly explain the age-related changes in epidural dose requirements [22].
The depth of the epidural space from the skin varies with body habitus, being less in a thin individual and more in an obese or pregnant individual [23, 24]. Ultrasound is a useful tool for measuring this depth and has been shown to correlate well with the actual depth [25].
Physiological Effects of Neuraxial Block
The physiological effects of both subarachnoid and an epidural block are quite similar. However, the effects of an epidural block have a slower onset and are usually segmental in nature, due to the segmental spread of local anesthetic in the epidural space. These effects are summarized below.
Neurological Blockade
The injection of local anesthetic within the intrathecal or epidural space produces nerve blockade. This blockade first affects the smaller-diameter sympathetic fibers, before the larger myelinated sensory–motor fibers [26, 27]. As a result, autonomic block manifests before sensory block, which in turn precedes the motor block. Among the sensory modalities, the sequence of blockade is temperature, pain, touch, pressure, and finally proprioception.
Block dissipation occurs in the reverse manner with autonomic fibers being the last to recover. In general, more dilute solutions affect the sensory fibers preferentially, while higher concentration is needed to block the motor fibers. Sensory block extends two to four segments higher than motor block, and the sympathetic block extends two to four segments higher than the sensory block. This is termed differential block [28].
Cardiovascular Effects
Blockade of the thoracolumbar sympathetic nerves manifests as the cardiovascular effects that follow a neuraxial block, including hypotension and bradycardia. These effects are in proportion to the extent of the block produced and may be exaggerated in a hypovolemic patient. Hypotension is a result of arteriolar vasodilatation and venous pooling which diminishes preload. Bradycardia is partially due to blockade of cardiac accelerator fibers (T1–T5) [29] but is more often a result of decreased preload which activates cardiac reflexes involving intracardiac stretch receptors [30]. The Bezold–Jarisch reflex in particular is often invoked as an explanation for severe bradycardia and asystole following spinal anesthesia in young healthy adults [31].
In general, the sympathetic block following a spinal anesthetic is more rapid and greater in extent compared to an epidural. Thus, a gradually dosed epidural may be useful in providing hemodynamic stability. Additionally, a high thoracic epidural block (T1–T4) provides coronary vasodilatation and reduces work of the myocardium by reducing afterload and reducing the heart rate [32]. However, this unopposed vagal tone has been thought to contribute to bradycardia, asystole, or laryngospasm [33, 34].
Respiratory Effects
In a patient with normal lung function, neuraxial blocks produce a clinically insignificant impact on respiratory function [35]. However, in high thoracic blockade or in a patient with preexisting respiratory compromise, this effect may be significant. The paralysis of abdominal and intercostal muscles negatively impacts expiratory function causing a reduction in peak expiratory flows, expiratory reserve volume, and maximal minute ventilation. This may manifest as dyspnea. However, these effects are negligible in comparison to the pain relief obtained following thoracic or abdominal surgery, and overall improvement in postoperative outcome and a reduction in postoperative pulmonary complications are observed [36].
Spread of the local anesthetics to cervical segments can affect inspiration by blocking the phrenic nerve and diaphragmatic function. Respiratory arrest following a total spinal may be due to medullary hypoperfusion rather than an effect of local anesthetics per se [35].
Gastrointestinal Function
The splanchnic blockade (T6–L2) produced following a neuraxial block leads to unopposed parasympathetic activity. This results in increased gastrointestinal secretions, increased intestinal motility, and relaxation of sphincters. This contracts the bowel and allows better access during abdominal surgery. Also, improved visceral perfusion due to vasodilation may improve healing and contribute to an earlier return of bowel function following surgery [37, 38].
Nausea and vomiting, if observed, are usually secondary to increased vagal tone and hypotension. A higher block (above T5), accompanying hypotension, use of opioids, and a history of motion sickness are risk factors for developing nausea and vomiting [39].
Genitourinary
Neuraxial anesthesia does not affect renal blood flow since it is autoregulated. Sacral blockade produces an atonic bladder and an increased bladder sphincter tone. This may result in urinary retention until the resolution of the block.
Thermoregulation
Neuroendocrinal Effects
Neuraxial block effectively blocks the afferent innervation from the surgical site and is responsible for the inhibition of the surgical stress response that involves release of a variety of mediators (including catecholamines, vasopressin, growth hormone, renin, angiotensin, glucose, antidiuretic hormone, and thyroid-stimulating hormone). This effect is greater for a lumbar epidural than a thoracic epidural [42]. The magnitude of the stress response correlates with postoperative morbidity, and thus, its attenuation should be of benefit [43].
Indications
Spinal Versus Epidural Anesthetic
Neuraxial techniques (spinal and epidural anesthesia) at the lumbar levels allow for a temporary blockade of nerve conduction in the autonomic, sensory, and the motor fibers. However, it is important to recognize differences between the two. These are summarized in Table 41.2.
Table 41.2
Important differences between a spinal and an epidural anesthetic
Variables | Spinal anesthetic | Epidural anesthetic |
|---|---|---|
Space accessed | Deposition of local anesthetic in subarachnoid space | Deposition of local anesthetic in epidural space |
Time needed to perform | Takes less time (since it is usually a single-shot technique) | Takes more time (since it is usually a catheter technique) |
Onset | Faster | Slower |
Nature of blockade | Denser, therefore mainly employed as an anesthetic technique | Less dense, therefore mainly employed as an analgesic technique. However, with the use of sufficient volume and concentration, an anesthetic block can be produced |
Duration | Dependent on dose and agent; fixed duration unless a catheter is inserted | Duration is flexible as a catheter is usually inserted |
Cardiovascular effect | Rapid drop in blood pressure | A slower drop in blood pressure |
Comparison to General Anesthesia
When compared to a general anesthetic, the central neuraxial techniques offer the following advantages:
Avoidance of airway manipulation
Avoidance of side effects of a general anesthetic (sore throat, nausea and vomiting, dental damage, malignant hyperthermia, aspiration of gastric contents, etc.)
Simple and reliable block
Predictable physiologic changes
Minimal metabolic disturbances (in hepatic or renal disease)
Preservation of consciousness
Extension into postoperative analgesia
When compared to general anesthesia, neuraxial techniques have been associated with a reduction in cardiovascular adverse events, perioperative pulmonary complications, thromboembolic events, preserved gastrointestinal motility, development of chronic pain, surgical stress response, immune dysfunction, and morbidity and mortality [44–48].
However, general anesthesia is advantageous over neuraxial block when better control over hemodynamics is desired (such as in severe or critical aortic stenosis). It offers definitive control of the airway and allows for immediate postoperative pain assessment.
Indications
The application of neuraxial anesthesia depends on the following factors:
The area of surgery
The type and expected duration of the procedure
The degree of muscle relaxation required
The presence of concomitant disease
The expected blood loss
The relevant indications for spinal and epidural anesthetic are considered in Table 41.3.
Spinal anesthetic | Epidural anesthetic |
|---|---|
Surgical | Surgical indications |
Spinal anesthesia is particularly advantageous for all types of surgical procedures below the level of the umbilicus | Procedures in the area of the lower extremities, hip joints, and inguinal region |
Surgical procedures in the area of the lower extremities, hip joint, and inguinal region | Vascular surgery |
Vascular surgery | Upper abdominal and thoracic procedures, in combination with general anesthesia |
Prostate and bladder surgery | Urological procedures (prostate, bladder) |
Gynecological and obstetric procedures | Gynecological and obstetric procedures |
Surgery in the perineal and perianal region | Procedures in the perineal and perianal region |
Pain therapy | Interventional radiology |
Chemical intraspinal neurolysis with phenol in glycerol or alcohol (in advanced stages of malignant disease) | Postoperative and post–traumatic pain therapy |
Usually in combination with local anesthetics and opioids | |
Therapeutic block with injection of depot corticosteroids at caudal, lumbar, or cervical levels | |
Epidural injection of homologous blood or dextran or fibrin glue patch in post–dural puncture headache |
Contraindications
These are summarized in Table 41.4.
Table 41.4
Contraindications to central neuraxial block
Absolute | Relative |
|---|---|
Patient refusal | Coagulopathy: coagulation disorders or anticoagulant therapy |
Sepsis, local infections at the injection site, or immune deficiency | Spinal pathology including severe spinal deformities, arthritis, osteoporosis, intervertebral disc prolapse, spinal canal stenosis, post-spinal surgery, and spinal metastases |
Severe decompensated hypovolemia, shock | Unknown duration of surgery |
Severe specific cardiovascular diseases of myocardial, ischemic, or valvular origin | Mild valvular disease (aortic stenosis) |
Acute cerebral or spinal cord diseases | Preexisting neurological deficits (radiculopathies, peripheral neuropathies, multiple sclerosis) |
Increased intracranial pressure | |
Indeterminate neurological disease | |
Hypersensitivity to local anesthetic agents |
Coagulopathy, whether iatrogenic or idiopathic, is now considered a relative contraindication. According to the consensus statements by the American Society of Regional Anesthesia and Pain Medicine (2010), epidural block can be placed 4 h after the last dose of heparin. NSAIDs including aspirin are not a contraindication to epidural placement provided that catheter placement is uncomplicated (one or two attempts). LMWH should be held at least 12 h before placement of catheter and 2 h after its removal. Epidural placement is relatively safe with international normalized ratio (INR) <1.5. If an epidural vein is punctured, subcutaneous heparin administration should be held at least 2 h and LMWH held at least 24 h. GIIa/IIIb inhibitors should be withheld for at least 4 weeks after epidural placement. Epidural placement is best avoided for 7 days after clopidogrel and 14 days after ticlopidine. For a further review on the use of neuraxial techniques in anticoagulated patients, patients on antiplatelet drugs, or patients with coagulopathy, please refer to the “Executive summary: regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition)” [49].
Performing a Spinal Anesthetic
Before performing a spinal anesthetic, a full discussion of the risk–benefit and informed consent should take place.
Preparation and Materials
Check that the emergency equipment is complete and in working order (intubation kit, emergency drugs); sterile precautions, intravenous access, and anesthetic machine.
Start an intravenous infusion and ensure adequate volume loading (250–500 ml of a balanced electrolyte solution).
Vasopressors such as ephedrine, phenylephrine, or metaraminol should be readily available.
ECG, noninvasive blood pressure, and pulse oximetry monitoring are essential.
Skin prep using an alcohol, iodine, or chlorhexidine. Note that if chlorhexidine is used, extreme care must be taken to prevent its inadvertent introduction into the neuraxis, as it has been implicated in chemical arachnoiditis [50, 51].
Local anesthetic for skin infiltration and intrathecal injection.
Spinal needles (Fig. 41.8a–c) are commonly of the following two types:
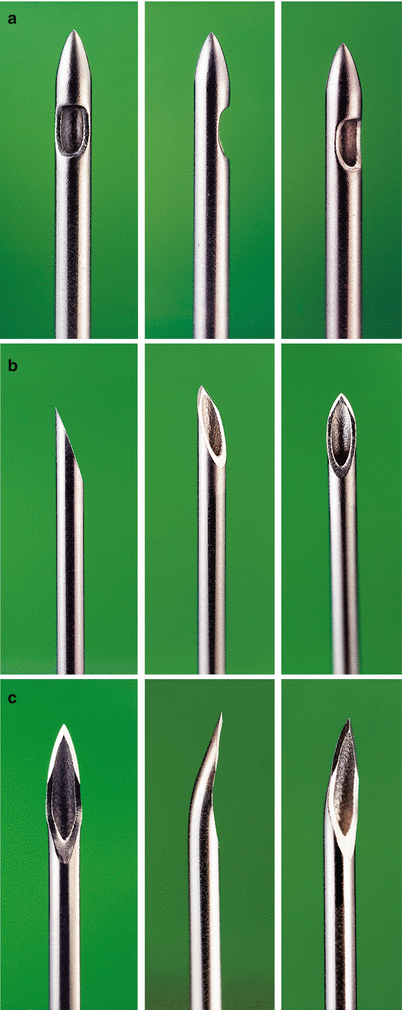
Fig. 41.8
(a–c) Spinal needles. (a) Pencil-point, 25 G. (b) Quincke tip, 27 G. (c) Atraucan Special Cut, 26 G (With permission from Danilo Jankovic)
25–27–G spinal needles with conical tip (pencil-point)—e.g., Sprotte, Pencan, and Whitacre—in current standard use. When the dura is penetrated with these needles, the dural fibers are separated and then closed together again. This reduces the risk of post-dural puncture headache [52, 53].
25–27–G spinal needles with Quincke tip. The needle bevel should be directed laterally during the puncture in order to pass through the dura in a longitudinal direction [54]. A 22-G needle may be used where increased stiffness is desired to facilitate needle redirection—e.g., in older patients with narrowed interlaminar spaces or when there are difficulties with positioning.
Patient Positioning
Optimal patient positioning during puncture and during the fixation phase of the local anesthetic is a prerequisite for successful spinal anesthesia. The following positions may be used:
Lateral decubitus position
Sitting
Prone
In all three positions, it is important to locate the midline and to follow it during the entire injection procedure. Lumbar lordosis must be minimized.
Lateral Decubitus Position
The assistant stands in front of the patient. If the anesthetist is right-handed, the patient is placed in the left lateral position. The patient is asked to adopt a hunchback position (legs flexed up against the abdomen and chin flexed down onto the chest) in order to flex the lumbar spine and allow optimal expansion of the intervertebral spaces. It is important here for the spine to be parallel and for the intercristal line and the line connecting the two scapular tips to be perpendicular to the operating table (Fig. 41.9).
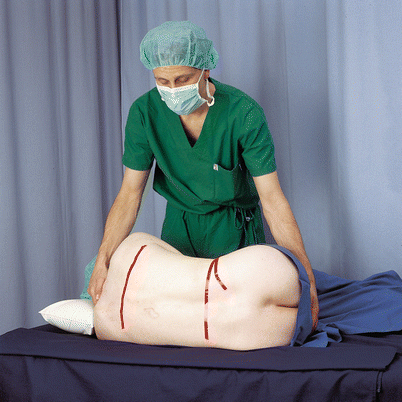

Fig. 41.9
Position: lateral decubitus (With permission from Danilo Jankovic)
Advantages
More comfortable for the patient and thus particularly suitable for frail patients (risk of collapse).
The reduction in blood pressure is less marked [55].
When hyperbaric solutions are used, unilateral anesthesia is more easily obtained.
Can be used in pregnant patients (since the lateral tilt or lateral decubitus position reduces the extent of aortocaval compression) [56].
Disadvantages
Difficulty may arise in the correct identification of the midline, especially if the pelvis is tilted too anteriorly.
Sitting Position
The patient is seated on the edge of the operating table and is supported by an assistant standing in front of him or her (Fig. 41.10). It may also be helpful to provide a padded Mayo stand, an adjustable table, or a similar device for the patient to lean on for additional support.
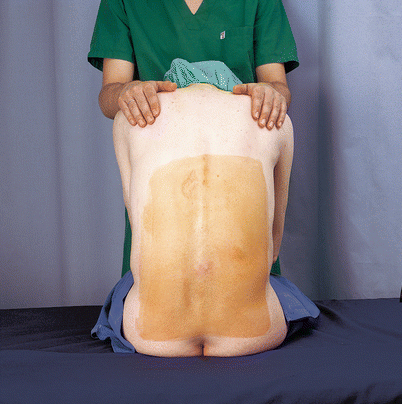

Fig. 41.10
Position: sitting (With permission from Danilo Jankovic)
Advantages
When palpation of the spinous processes is difficult (e.g., in obese patients or those with spinal deformities), it is easier to locate the midline in the sitting position.
Facilitates the performance of a saddle block with hyperbaric local anesthetic when anesthesia in only the perineal or perianal region is required.
Disadvantages
This position may lead to orthostatic hypotension (and risk of collapse) and should be avoided in frail and heavily sedated patients [55].
More support and assistance may be required to keep patient upright.
Prone Jackknife
This position is only used in the very rarely practiced hypobaric technique for spinal anesthesia (procedures in the rectum, perineum, sacrum, lower spine) [57]. An assistant and subsequent repositioning of the patient are not required (Fig. 41.11).
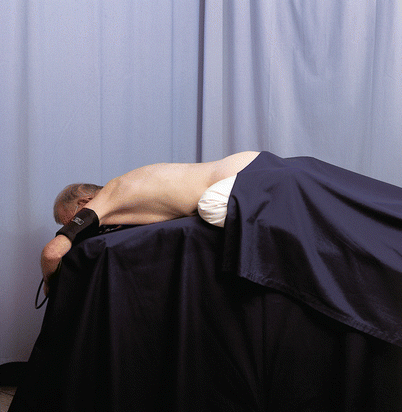

Fig. 41.11
Position: prone (“jackknife” position) (With permission from Danilo Jankovic)
Injection Technique
The subarachnoid space may be accessed using a midline approach, a paramedian approach, or a Taylor’s approach. These are discussed below.
Median Approach (Midline)
Landmarks
The injection is carried out in the midline below the L2 segment (conus medullaris), usually between the spinous processes of L2/L3 or L3/L4 (depending on the desired level of anesthesia). The patient is asked to draw the legs tightly up to the abdomen and to place the chin on the chest. A line is drawn from one iliac crest to the other. This connection (Tuffier’s line) crosses either the spinous process of L4 (50 %) or the intervertebral space of segments L4/L5. However, this palpatory method alone may be unreliable, and ultrasound may help to correctly identify the vertebral levels [7].
The spinous processes and intervertebral spaces are palpated to identify the midline, which is the important landmark. It is recommended that the index and middle fingers of the nondominant hand be used to palpate and identify the chosen interspace, as they can be subsequently used to fix the overlying skin, which is important for precision and accuracy in needle insertion (Fig. 41.12).
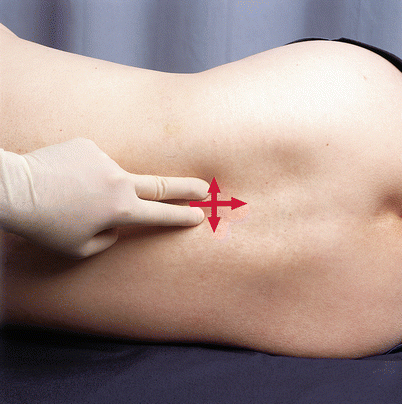

Fig. 41.12
Palpation of the intervertebral space (With permission from Danilo Jankovic)
Strict Asepsis
Thorough, repeated, and wide skin prep and drying and covering of the injection site with a drape.
Local Anesthesia
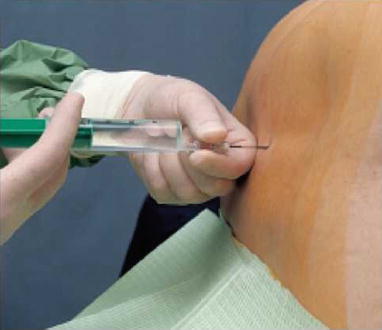
The skin and supraspinous and interspinous ligaments are anesthetized with 1–1.5 ml of a local anesthetic (e.g., 1 % lignocaine). The injection is carried out between the spread index and middle fingers of the left hand (Fig. 41.13). In the patient with less-distinct surface landmarks, the local anesthetic needle may be used to explore the underlying anatomy. Bony contact at a relatively shallow depth indicates contact with the tip of the spinous process and the location of the midline. Firm resistance to further injection of local anesthetic indicates that the needle tip lies within the interspinous ligament in the midline and not in the paraspinous muscles on either side.
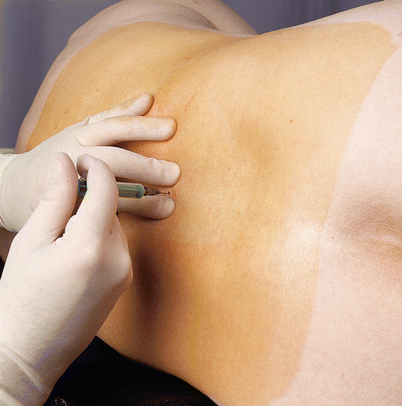

Fig. 41.13
Local anesthesia (With permission from Danilo Jankovic)
Injection
Advancing the Introducer
Without moving the spread index and middle finger of the left hand away from the intervertebral space (which prevents inadvertent movement of the skin overlying the chosen interspace), the introducer is grasped between the thumb and index finger of the right hand and advanced parallel to the operating table and slightly cranially (10°) deep enough for it to sit firmly in the interspinous ligament (Fig. 41.14). It should be ensured that the midline position is maintained. After this, the introducer is fixed with the thumb and index finger of the left hand, with the dorsum of the hand lying firmly on the patient’s back.
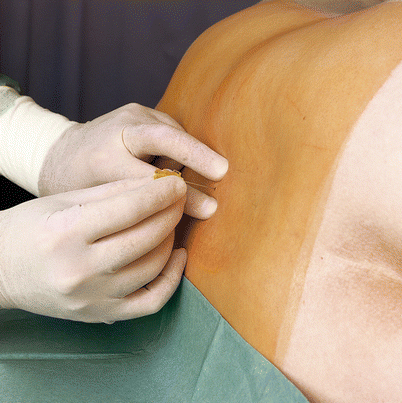

Fig. 41.14
Advancing the guiding cannula (With permission from Danilo Jankovic)
Introducing the Spinal Needle, Puncture of the Subarachnoid Space
The spinal needle, held between the thumb and index finger (or middle finger) of the right hand, is introduced through the interspinous ligament, ligamentum flavum, epidural space, and dura/arachnoid as far as the subarachnoid space. Penetration of the ligamentum flavum is usually evident by the “rubbery” resistance to needle advancement. A characteristic “dural click” may be felt when the subarachnoid space is reached; however, this does not always occur (Fig. 41.15). When a Quincke needle is used, it should be ensured that the needle bevel is directed laterally, so that the dura is punctured in a longitudinal direction.
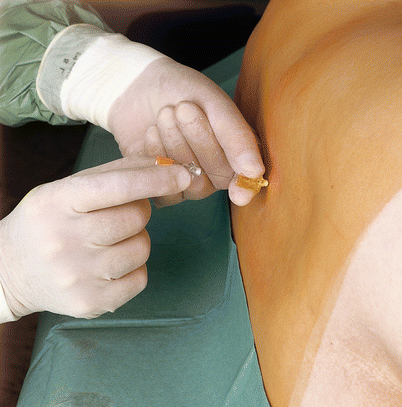

Fig. 41.15
Introducing the spinal needle. Puncture of subarachnoid space (With permission from Danilo Jankovic)
Removing the Stylet
The following may occur here:
CSF flows freely
The injection needle is advanced 1 mm and fixed between the thumb and index finger of the left hand, which is supported on the patient’s back. The desired amount of local anesthetic can now be injected (Figs. 41.16 and 41.17a, b).
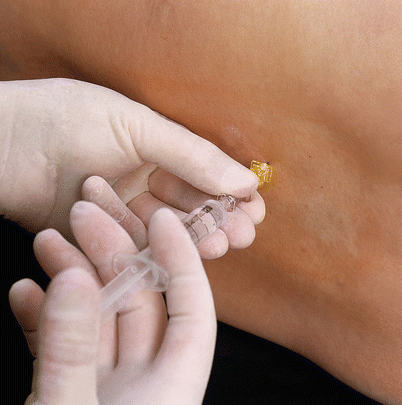
Fig. 41.16
Removing the stylet. Subarachnoid injection (With permission from Danilo Jankovic)
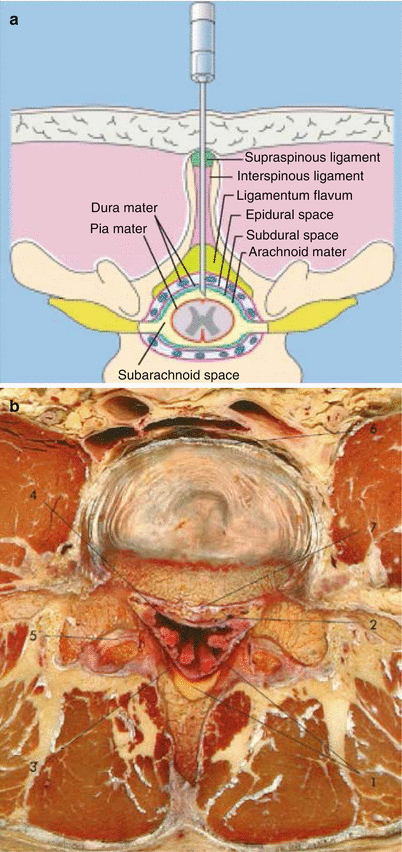
Fig. 41.17
(a) Subarachnoid position of the needle (With permission from Danilo Jankovic). (b) Transverse dissection at the level of L4/L5. (1) Posterior epidural space with fat, (2) anterior epidural space with veins, (3) spinal dura mater, (4) subarachnoid space and cauda equina, (5) zygapophysial joint, (6) anterior longitudinal ligament, and (7) posterior longitudinal ligament (With permission from Danilo Jankovic)
With the single-injection technique, aspiration of CSF (0.1 ml) should be attempted immediately before and after injection of local anesthetic. The subarachnoid injection is made at the rate of 1 ml per 5 s.
Blood in the CSF
Slightly bloody CSF which clears quickly (spontaneously or after aspiration) usually occurs after penetration of an epidural vein on the way into the subarachnoid space. The local anesthetic can be injected.
However, backflow of frank blood indicates that the injection needle is likely positioned within a vein. A new attempt at puncture must be made and possibly in a different intervertebral space.
No CSF flow
Rotation of the needle to all four quadrants and careful aspiration may help. The needle may also be advanced slightly with the stylet in place.
If no CSF flows in spite of all these measures, the needle should be removed and the procedure repeated with a different needle direction or at a different interspace.
Unexpectedly deep bone contact suggests that the posterior side of the vertebra or an intervertebral disc has been reached. CSF appears in most cases after the needle has been slightly withdrawn and aspiration has been repeated.
Pain or paresthesias during puncture
Pain or paresthesia prior to entry into the epidural or intrathecal space is most often due to needle contact with the facet joint, which forms the lateral border of the interlaminar space. The needle should be directed slightly more medially, away from the side on which the pain or paresthesia occurred. It is not uncommon for a transient paresthesia radiating down one leg to occur upon needle entry into the intrathecal space; this signals contact with the cauda equina. If it persists, the needle must be withdrawn slightly or repositioned. When paresthesias occur during the injection, the needle must be repositioned before any further drug is injected.
The local anesthetic must never be injected without evidence of CSF! The location and distribution of paresthesias arising during the puncture procedure must be recorded.
Experience shows that failure is usually due to deviation of the needle from the midline or excessive cranial angulation of the needle.
Paramedian Approach (Lateral, Paraspinal)
In this technique (Figs. 41.18 and 41.20), the supraspinous and interspinous ligaments are avoided, so that the ligamentum flavum is the primary target on the way to the subarachnoid space.

Fig. 41.18
Paramedian approach (With permission from Danilo Jankovic)

Fig. 41.19
Taylor’s approach (With permission from Danilo Jankovic)

Fig. 41.20
Puncture of the subarachnoid space: (1) median, (2) paramedian, (3) Taylor (With permission from Danilo Jankovic)
Procedure
This technique can be used in all the patient positions mentioned above. Flexion of the spine is not essential. The caudal edge of the spinous process is marked. The injection site is located 1–1.5 cm lateral and caudal to this. The puncture is carried out in a cranio-medial direction, at an angle of about 10–15°. The dura is reached after about 4–6 cm. Most mistakes arise when the needle is angled too cranially or when the needle entry point is too lateral.
This technique can be used in:
Degenerative changes in the spine
Older patients with marked calcification of the supraspinous and interspinous ligaments [58]
Obesity
Fractures or other pathological conditions in which pain makes it impossible to flex the spine
Taylor’s Approach
This lumbosacral approach (Figs. 41.19 and 41.20) is a paramedian injection via the intervertebral space of L5 and S1, the largest interlaminar space in the spinal region.
Procedure
The injection site is located about 1 cm medial and about 1 cm caudal to the posterior superior iliac crest. The injection needle is advanced in a cranio-medial direction and at an angle of about 55°. If it touches the periosteum (sacrum), the needle must be withdrawn and its direction must be corrected.
The indications for this access route include procedures in the perineal and perianal region, as spread to the higher spinal levels may not always reliably occur.
Unilateral Spinal Anesthesia
Unilateral spinal anesthesia is intended to block only the anterior and posterior spinal nerve roots on the side being operated on, while the contralateral side—and particularly its sympathetic fibers—remains unblocked. This leads to a reduced incidence of hypotension.
Indications
Surgical and orthopedic procedures on the lower extremities
Procedure
Patient positioning: lateral decubitus position, lying on the side that is to be operated on.
Injection technique: this is the same as for conventional spinal anesthesia. The opening of the pencil-point needle is rotated to the operating side, and the desired amount of local anesthetic is slowly injected [59]. A hyperbaric solution of local anesthetic such as bupivacaine should always be used for maximum effectiveness.
Patient position after the injection: the patient remains lying on the side that is to be operated on for about 20 min.
Advantages
Reduction in the extent of the sympathetic block (by about 70 %), since smaller volumes and slower injection of the local anesthetic mean that fewer spinal segments are involved [60]
Hemodynamic stability (hypotension is only observed in 5 % of patients)
Faster recovery from anesthesia
Suitable for outpatient procedures [61]
Greater acceptance by patients
Disadvantage
Strict unilateral anesthesia is only achieved if adequate time is allowed for the local anesthetic to settle on the operative side. However, a differential block of some degree can almost always be achieved.
Continuous Spinal Anesthesia
The insertion of a catheter into the subarachnoid space allows a continuous or repeated intermittent dosing of local anesthetic.
Indications
Lower abdominal and lower limb surgery in elderly patients and high-risk patients [62]
Postoperative pain relief
Chronic pain relief (in cancer patients)
Procedure
The procedure is similar to a standard spinal anesthetic. However, usually a 20–22-G spinal needle is used to gain access to the subarachnoid space, and a 25–27-G catheter is threaded (usually 2–3 cm) into the subarachnoid space (Fig. 41.21).
Fig. 41.21
Introducing the catheter in the subarachnoid space (With permission from Danilo Jankovic)
Advantages
A smaller initial dose of local anesthetic can be injected without concern about inadequate block height or duration. This helps prevent hypotension due to excessive sympathetic block caused by large doses or greater spread of local anesthetic [62].
Ability to prolong spinal anesthetic as needed.
Management of the Patient After Intrathecal Injection
Patient Positioning
The level of the anesthetic spread is controlled by patient positioning measures and checked with cold tests at intervals of 2–5 min.
Hyperbaric Spinal Anesthesia
Lateral decubitus position: the patient remains on the side of surgery for 10–15 min if unilateral anesthesia is desired. The patient is laid supine if bilateral anesthesia is required.
Sitting position: the patient is immediately laid supine to allow the anesthetic to spread cephalad. The patient remains sitting if sacral spread is desired.
Hypobaric Technique
Hypobaric spinal anesthesia is ideal for perineal and perirectal surgeries requiring a prone “jackknife” position, so that the patient does not need to be repositioned (Fig. 41.11).
The hypobaric technique has also gained popularity in major hip surgery, which is performed in the lateral position. Thus, repositioning of the patient is often not needed when the spinal is placed in the lateral decubitus position, with the operative side nondependent. Compared to isobaric bupivacaine, a hypobaric solution demonstrated a significant delayed block recession and time until analgesia, for total hip arthroplasty [65].
Isobaric Spinal Anesthesia
Horizontal positioning is adequate; other positions have no significant influence on the spread of the anesthesia.
Fixation Phase
The phase immediately after injection of the local anesthetic is particularly critical and requires precise monitoring. The fixation phase lasts about 10–15 min.
Patient Monitoring
Sympathetic block following a spinal anesthetic results in a drop in the blood pressure and heart rate. This may be profound in elderly and volume-depleted patients. Bradycardia may occur after the peak of sympathetic block is achieved (30–60 min after spinal injection) due to the blockade of cardiac accelerator fibers. Unexpected bradycardia and cardiac arrest may occur in young healthy patients [66]. Thus, frequent monitoring of the vitals (heart rate, blood pressure, and oxygen saturation) is advocated during and after the conduct of a spinal anesthetic.
Supplemental oxygen administration is recommended after a spinal anesthetic, especially if sedation is used for patient comfort.
End-tidal carbon dioxide monitoring is also used to monitor the respiratory rate.
Postoperatively, the patient should be monitored until the effects of spinal anesthetic have receded.
Block Assessment
Injecting a local anesthetic into the subarachnoid space blocks autonomic, sensory, and motor function. The main targets of local anesthesia are the posterior roots with the ganglia, the anterior roots of the spinal nerves, the autonomic nerve fibers, and mixed neural trunks.
The spread of the anesthesia should be checked at short intervals (2–5 min) and confirmed (with a cold spray or pinprick) shortly before the start of the operation [67, 68]. The first sign of an effect on the spinal nerve roots is a subjective sensation of warmth in the feet. The further development of the block encompasses touch, deep pressure, motor function, vibration sensitivity, and positional sense.
Motor function is completely blocked at the site of the greatest concentration of the local anesthetic. Sensory block extends two to four segments higher than motor block, and the sympathetic block extends for a further two to four segments higher than the sensory block.
Resolution of the block is marked by a return of motor function, followed by the sensory modalities. The autonomic function is the last to recover [28].
Performing an Epidural Anesthetic
Preparation and Materials
Preparation for an epidural anesthetic is similar to that for a spinal block. This has already been discussed in Sect. Patient Positioning.
Epidural needles: The epidural needles required to perform an epidural block are generally larger in gauge than spinal needles. Additionally, they may have distinct tip design to facilitate entry into the epidural space and introduction of the catheter. Some of these are already discussed in Sect. Patient Positioning. Of these, Tuohy-tip epidural needles are most commonly used.
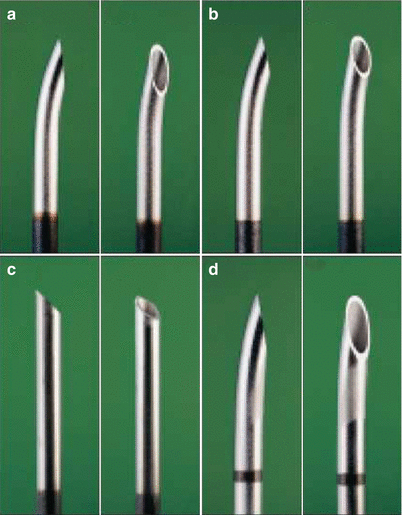

Fig. 41.22
(a–d) Epidural needles. (a) Tuohy, (b) Hustead, (c) Crawford, (d) Weiss (With permission from Danilo Jankovic)
Positioning of the Patient
An epidural block can be performed with the patient in lateral decubitus or sitting position. While the former is commonly used in obstetric patients, the latter is preferred in other patient groups. Prone position is rarely used. The pros and cons of these positions have been already discussed on p. 514 (Patient Positioning).
Performing a Lumbar Epidural
Lumbar epidural space may be accessed using a midline approach which is most commonly used or using a paramedian approach. These are discussed below.
Midline Approach
Landmarks
The injection is carried out in the midline below the L2 segment (conus medullaris) (Fig. 41.23a, b), usually between the spinous processes of L2/L3 or L3/L4. The intervertebral space is palpated, and the midline is located to serve as the most important signpost. In the midline, the ligamentum flavum is at its thickest, the epidural space is widest, and the blood vessels are at their smallest [69].
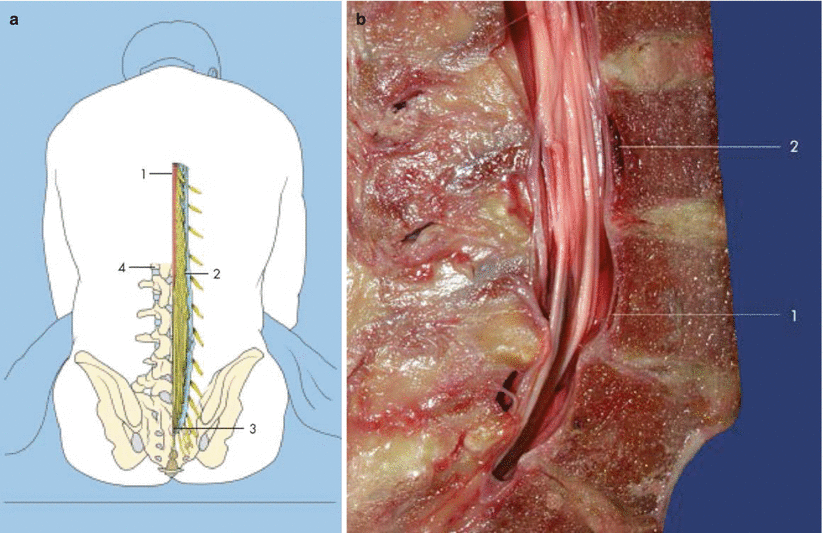

Fig. 41.23
(a) Conus medullaris (lower edge of the first lumbar vertebra). (1) Conus medullaris, (2) cauda equina, (3) dural sac, (4) L1 segment (With permission from Danilo Jankovic). (b) Cauda equina at the level of L2–L5. Paramedian sagittal section. (1) Spinal dura mater, (2) epidural space (With permission from Danilo Jankovic)
Strict Asepsis
Thorough, repeated, and wide skin prep is done. After drying, the injection site is covered using a sterile drape.
Local Anesthesia
The skin and supraspinous and interspinous ligaments are anesthetized with 1–1.5 ml of a local anesthetic (e.g., 1 % lignocaine). The injection is carried out between the spread index and middle fingers of the left hand (Fig. 41.24).

Fig. 41.24
Local anesthesia (With permission from Danilo Jankovic)
Needle Insertion
Without moving the spread index and middle fingers of the left hand from the intervertebral space, an epidural needle is fixed between the thumb of the right hand (hub) and the index and middle finger (shaft) and advanced through the skin (Fig. 41.25). After passing the supraspinous ligament, which is about 1-cm thick, the needle, with its bevel directed cephalad or caudad, is slowly advanced a further 2–3 cm (depending on the anatomy), until it rests firmly in the interspinous ligament. This often results in a “gritty” sensation. The trocar is removed and a low-friction (loss of resistance) syringe is attached (Fig. 41.26).

Fig. 41.25
Introducing the epidural needle (With permission from Danilo Jankovic)

Fig. 41.26
Removing the stylet and attaching a low-friction syringe (With permission from Danilo Jankovic)
Care should be taken to ensure that the needle is kept in the midline. Inadvertent deviation from the midline leads to the needle passing the supraspinous ligament, with an angled entry into the interspinous ligament with only brief resistance and a subsequent false loss of resistance. This type of puncture ends in the paravertebral musculature and is accompanied by local pain.
After passing the interspinous ligament, the needle must be advanced carefully, millimeter by millimeter, in the direction of the ligamentum flavum.
Puncturing the Epidural Space
The thumb and index finger of the left hand, which is resting with the back of the hand firmly against the patient’s back, secure the needle, advance it millimeter by millimeter, and at the same time serve as a “brake” to prevent inadvertent forward movement. The thumb of the right hand applies pressure on the syringe plunger. Loss of resistance indicates that the epidural space has been reached. The contents of the syringe are easily injected.
Identification of the epidural space is carried out using the loss–of–resistance technique (Fig. 41.27). The following variations on this technique can be applied:

Fig. 41.27
Identifying the epidural space (loss of resistance) (With permission from Danilo Jankovic)
(a)
Saline with an air bubble: after the interspinous ligament has been reached, the stylet is removed, and a low-friction syringe filled with a saline solution and with a small air bubble in it, serving as a visual indicator, is attached. When the ligamentum flavum is encountered, the air bubble is compressed by pressure on the syringe plunger (Fig. 41.28a); when the epidural space is reached, the bubble returns to its normal, larger shape (Fig. 41.28b). This technique is the most common choice as it provides a good identification of the epidural space and an idea of the force being applied (by the compression of the air bubble).
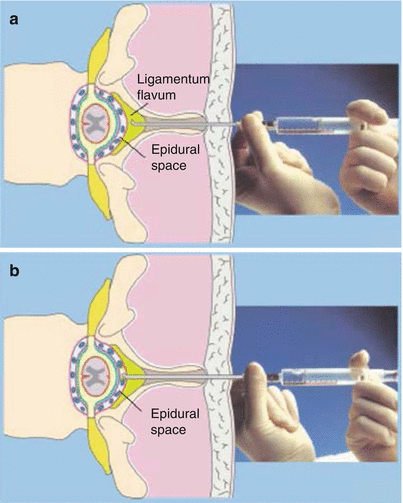

Fig. 41.28
(a) Loss-of-resistance technique with saline. The air bubble is compressed by pressure on the syringe plunger (With permission from Danilo Jankovic). (b) Loss-of-resistance technique with saline. The epidural space has been reached. The air bubble has returned to its normal, loose shape (With permission from Danilo Jankovic)
(b)
Saline–only technique: since the introduction of air in the epidural space may lead to “patchy” block, some clinicians prefer to use only saline. The disadvantage of doing so is the inability to objectively judge the amount of force being applied on the plunger.
(c)
Air–only technique: this technique allows for a more subjective “feel” while identifying the epidural space. It also allows clearer identification of an accidental dural puncture as any fluid that emerges from the hub of the needle must be CSF. However, using air to locate epidural space has been associated with patchy blocks, venous air embolism, and pneumocephalus [70].
(d)
Hanging drop technique: in this technique, after the interspinous ligament has been reached, a drop of saline is placed within the hub of the needle (Fig. 41.29a). After the ligamentum flavum has been passed and the epidural space has been reached, the drop is “sucked in” due to the negative pressure in the epidural space (Fig. 41.29b) [71].
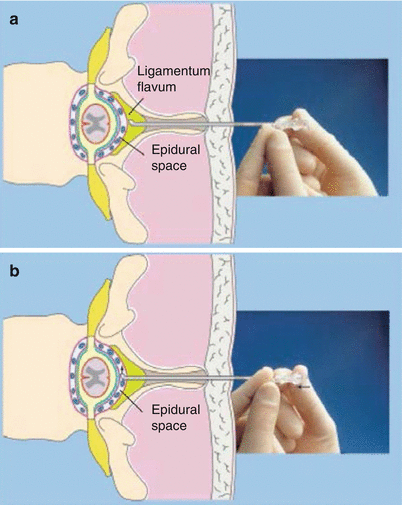

Fig. 41.29
(a) “Hanging drop” technique. The epidural needle is positioned in the ligamentum flavum (With permission from Danilo Jankovic). (b) “Hanging drop” technique. The epidural space has been reached. The drop is sucked back in (With permission from Danilo Jankovic)
Aspiration and Injection of a Test Dose
Having reached the epidural space, a careful aspiration is carried out to detect CSF or blood while the needle continues to be secured by the thumb and index finger of the left hand, resting with the back of the hand firmly against the patient’s back (Fig. 41.30).
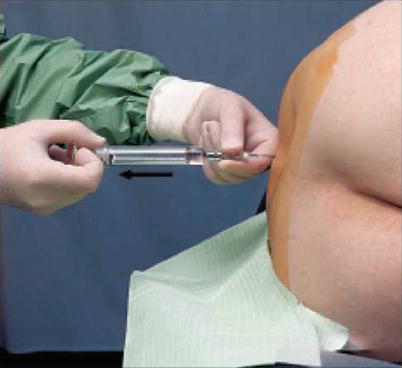

Fig. 41.30
Aspiration test (With permission from Danilo Jankovic)
After a negative aspiration, a test dose of 3–4 ml of local anesthetic (usually 1.5 % lignocaine) and 15 mcg epinephrine can be injected. This allows detection of intrathecal injection (rapid development of spinal block) or intravascular injection (tachycardia).
The addition of epinephrine can lead to unreliable results in patients taking beta-blockers, patients under general anesthesia, and pregnant patients. Due caution must be exercised while adding epinephrine in pregnant patients (may cause transient fetal bradycardia due to reduced uterine blood flow), older patients with coronary artery disease, and hypertensive patients. The use of epinephrine is best avoided in patients with closed-angle glaucoma and tachyarrhythmia [72].
Maintaining constant verbal contact with the patient and careful cardiovascular monitoring after a test dose is recommended. If all is well, a further dose of the local anesthetic can be injected.
Drug Administration
The epidural injection may be performed as a single-shot injection or as a catheter technique (allowing further dosing).
Get Clinical Tree app for offline access

(a)
Single–injection technique: incremental administration of a local anesthetic is carried out in aliquots of 3–5 ml, waiting 15–20 s between each dose, with intermittent aspiration (Fig. 41.31). After aspiration has been repeated shortly before the end of the injection, the needle is withdrawn and the patient is placed in the desired position.