Fig. 4.1
Ulnar nerve stimulation. Electron flow is toward the needle with the needle as the cathode. This causes an area of depolarization around the needle tip and a subsequent action potential causing a motor response
Needles used for nerve stimulation are insulated with a nonconducting material. This directs the current density to a sphere around the uncoated needle tip. The use of nonelectrolyte/nonconducting injectates, for example, dextrose 5 % in water (D5W), reduces the conductive area around the needle tip and increases the current density resulting in maintenance or even augmentation of the motor response at a low current (<0.5 mA).
Important Adjustable Features of Modern Nerve Stimulators
Current: Coulomb’s law describes the relationship between distance and current intensity:
I current required, k constant, i minimal current, r distance from nerve
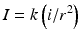
Consequently, as the distance between needle and nerve decreases, a lower current intensity should be required to initiate a motor response.
Pulse width: The duration of the pulse enables selective stimulation of sensory or motor nerves. Motor nerves are more easily targeted with shorter pulse widths (e.g., 50–150 μs).
Frequency of stimulation: Low frequencies may cause the target nerve to be missed due to poor timing. Most operators utilize a frequency of 2 Hz.
Concerns Regarding Nerve Stimulation in the Ultrasound Era
Ultrasound imaging, while having enhanced our understanding of the needle–nerve relationship, has created ambivalence regarding long-held tenets of nerve stimulation. As we have seen in the above section, as distance between the needle and nerve decreases, a lower current intensity should be required to initiate a motor response. However, when observed under ultrasound guidance, a motor response to nerve stimulation is frequently not seen until the needle tip is advanced into an intraneural location [3]. On occasion, with an intraneural needle tip location, a high stimulating current may be required to generate a motor response. A stimulating current as high as 1.5 mA has been found not to produce a motor response when the needle tip is located in the intraneural space. This contradicts previously held electrophysiological principles upon which safe practice in peripheral nerve blockade was based. Current evidence suggests that a motor response to a stimulating current of 0.2 mA always signifies an intraneural position [4, 5]. The distillation of human and animal studies into clinically useful guidelines advocates that nerve stimulation has higher specificity than sensitivity for detecting intraneural needle placement. In other words, a response to stimulation at a low current (≤0.2 mA) confirms intraneural placement, but lack of a response does not necessarily rule it out.
Mechanisms of Nerve Injury: Is It Possible to Detect Intraneural Injection Using Ultrasound?
To answer this important question, it is first necessary to review the basic histology of the nerve fiber. The axon is a projection of the nerve cell body. Electrical impulses are conducted through the axon away from the cell body. Axons may be coated with a myelin sheath which increases the speed of conduction. A peripheral nerve is composed of multiple nerve axons arranged into fascicles. Neural microarchitecture consists of a complex network of nerve tissue enclosed within concentric layers of protective connective tissue (Fig. 4.2). Individual axons are surrounded by a connective tissue layer called the endoneurium. The fascicle itself is enclosed in a tough and mechanically resistant sheath called the perineurium. A collection of fascicles, along with blood supply and fatty tissue, is surrounded by a third, and final, layer of connective tissue comprised of collagen and adipose tissue called the epineurium.
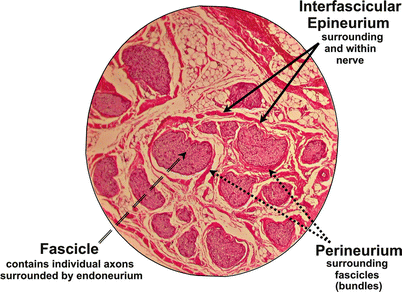

Fig. 4.2
Cross section of a nerve at 25× magnification. Fascicles are surrounded by protective connective tissue layers (perineurium and interfascicular epineurium). Note that the endoneurium is too fine to be seen with light microscopy at this magnification
The ratio of fascicular-to-epineurial tissue varies between 30 and 70 % of the total nerve area [6], a relationship which deviates not only between different nerves but also along individual nerves. The ratio of neural-to-nonneural tissue is greater closer to the nerve root for both the sciatic nerve and the brachial plexus, i.e., more fascicular tissue relative to surrounding connective tissue at the proximal sciatic nerve and interscalene brachial plexus, respectively [7, 8]. Fortunately, the path of least resistance for an intraneurally placed needle may be through the more compliant adipose tissue of the interfascicular epineurium rather than through the fascicles. However, significant and lasting nerve injury is thought to occur only when injection of solution occurs inside the fascicle.
Ultrasonographic detection of intraneural injection is largely dependent on the surrogate measure of nerve expansion upon injection. Indeed, ultrasonographic nerve expansion has been equated with intraneural injection as confirmed by histologic analysis in porcine studies [9, 10]. However, while ultrasound guidance may permit a rudimentary assessment of nerve diameter, the prohibitive resolution of available ultrasound technology precludes consistent differentiation between intrafascicular and extrafascicular injection. A 15-MHz transducer, in the high end of most practitioner’s armamentarium, only permits visualization of one-third of sciatic nerve fascicles as compared with light microscopy [11]. Regardless of its technological limitations, ultrasound images must be interpreted by the operator. Ultrasonographic evidence of nerve expansion may not always be obvious, and two recent cautionary case reports regarding brachial plexus blockade by experienced practitioners are testament to the imperfections of the technology [12, 13].
The overall incidence of late neurologic deficit is such a rarity that it precludes statistical substantiation by randomized controlled trials. Nevertheless, it is instructive to scrutinize the incidence of serious nerve injury associated with regional anesthesia before and after the introduction of ultrasound guidance. In one of the largest reports to date, a prospective survey in France recorded an incidence of late neurologic injury of 0.2/1,000 in over 150,000 regional anesthesia procedures [14]. In the ultrasound era, the Australasian Regional Anesthesia Collaboration reports a similar incidence of late neurologic deficit of 0.4/1,000 [15].
There is no evidence to suggest that nerve stimulation is any better at detecting intrafascicular needle placement than ultrasound guidance. However, the two modalities may be complimentary and serve to compensate for their respective deficits.
The authors, when performing blocks under ultrasound guidance, use a dual ultrasound–nerve stimulation technique. A constant stimulating current of 0.25 mA is used. This ensures patient comfort while improving the safety of nerve localization.
Nerve Stimulation and the Potential for Patient Injury
There appears little doubt than when electrical nerve stimulation is used as a location device in conjunction with ultrasound guidance that block performance times are lengthened without an improvement in success rates [16, 17]. This suggests a greater number of needle passes and associated patient discomfort. Moreover the lower dose and volume of local anesthetic solution permitted by the highly accurate perineural placement of local anesthetic solution under ultrasound guidance alone results in a significant reduction of local anesthetic systemic toxicity [2].
A longer block performance time may indeed be required if an anatomic and neurophysiologic endpoint are sought. However, when compared with an ultrasound guidance technique alone, the procedure time may be equivalent when using a dual ultrasound–nerve stimulation approach for the exclusion of intraneural needle placement rather than for confirmation of nerve location.
Use of Nerve Stimulation for Training Novices
While expertise in recognition and location of the pertinent sonoanatomy can be procured with time, haptic perception and consistent hand–eye coordination are more challenging skills to acquire. Failure to maintain needle tip visualization is the most common error observed in residents learning ultrasound-guided regional anesthesia [18]. Other common sources of error during novice practice and beyond include failure to appreciate the nuances between acoustic artifact and nerve and failure to distinguish between adjacent isoechoic structures, e.g., tendon and nerve.
The use of a dual nerve stimulation–ultrasound technique may improve block efficiency and efficacy while preventing injection of local anesthetic at a nonneural location. Even the experienced practitioner may benefit from the reassurance provided by nerve stimulation when faced with a challenging obese patient where target neural structures may be difficult to identify with precision at a deep location.
Stimulating Catheters
Peripheral Nerve Blocks
When compared with a single-shot technique, continuous regional anesthesia has the potential to improve the quality and duration of analgesia [19] and to cause fewer systemic side effects including local anesthetic toxicity. In addition, it may be associated with a less profound motor block and improved functional recovery. However, catheter techniques may be technically difficult and subsequently are subject to either primary or secondary failure, the latter occurring when the catheter tip is dislodged, either partially or fully, so that it is no longer in proximity to the nerve target. Historically, nerve block catheters were placed by identifying the neural target using an insulated stimulating needle through which a catheter was blindly threaded a variable distance with the expectation that it would follow the path of the nerve. A less arbitrary method involves the insertion of a perineural catheter which conducts current to its tip, the stimulating catheter. Though some studies do report a reduction regarding the need for rescue analgesia with stimulating catheters, results are not as consistent as expected with respect to pain scores and functional recovery [20].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








