FIGURE 6-1 Gross anatomy of the liver. (From Moore KL, Agur AMR, Dalley AF. Clinically Oriented Anatomy. 7th ed. Philadelphia: Wolters Kluwer; 2013, with permission.)
Key related structures of the hepatobiliary system include the gallbladder and its cystic duct outflow tract, which combines with the common hepatic duct to form the common bile duct. More distally, the common bile duct joins the pancreatic duct to form the hepatopancreatic ampulla (ampulla of Vater), which then drains bile and pancreatic secretions into the second portion of the duodenum through the sphincter of Oddi (Figs. 6-1 and 6-2).
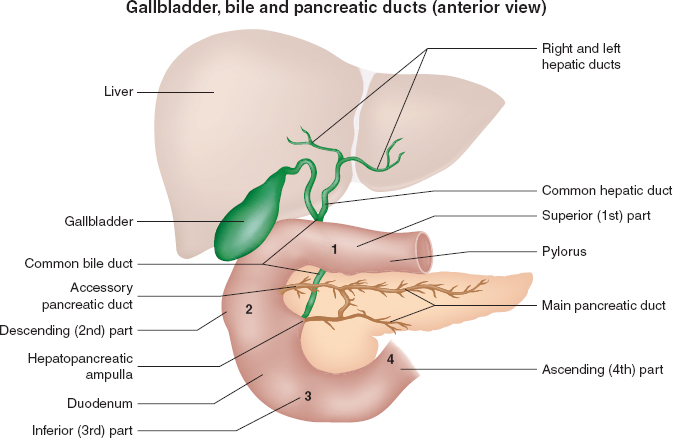
FIGURE 6-2 Gallbladder, bile, and pancreatic ducts. (From Moore KL, Agur AMR, Dalley AF. Clinically Oriented Anatomy. 7th ed. Philadelphia: Wolters Kluwer; 2013, with permission.)
II. Microscopic Anatomy
Hepatocytes are arranged within hepatic sinusoids surrounding a central hepatic vein and are bordered by interlobular portal triads consisting of a biliary duct, hepatic artery, and portal vein (Figs. 6-3 and 6-4). This functional anatomy results from a complicated embryologic ballet in which the growing organ forms around portal veins, with bile ducts originating out of precursor ductal plates situated on the portal veins. Hepatic sinuses run from the peripheral portal triads to the centrolobar hepatic veins and are lined with fenestrated endothelial cells, featuring intracytoplasmic pores and loose intercellular junctions. In addition to the endothelial cells, other resident cell populations of the sinusoidal wall include Kupffer cells (macrophages), stellate cells (responsible for extracellular matrix production and capable of contractile function to regulate sinusoidal blood flow), and pit cells (lymphocytes). The space separating the sinusoids from hepatocyte bars is known as the perisinusoidal space of Disse, and it contains extracellular matrix generated by stellate cells, Kupffer cells, and dendritic cells. These latter two cell types are involved in microbial and antigen host defense and contribute to the significant immune function of the liver as well as to the fibrosis observed with hepatic cirrhosis.
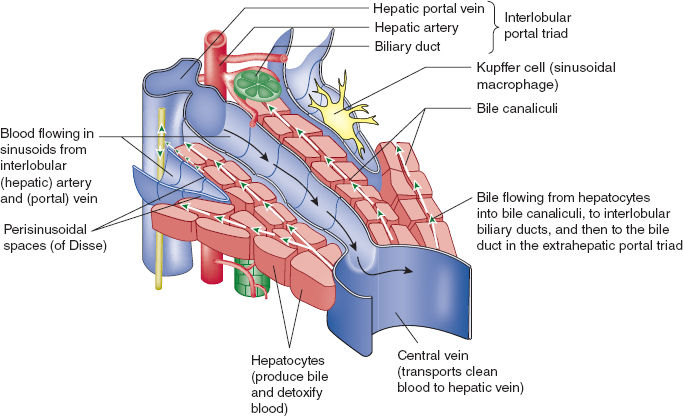
FIGURE 6-3 Section of hepatic lobule with functional diagram of bile and blood flow. (From Moore KL, Agur AMR, Dalley AF. Clinically Oriented Anatomy. 7th ed. Philadelphia: Wolters Kluwer; 2013, with permission.)
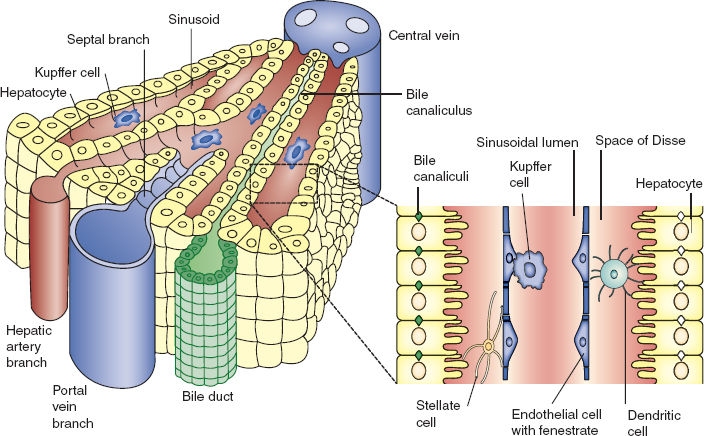
FIGURE 6-4 Portion of hepatic lobule: Microanatomy. (From Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nature Rev Immunol. 2006;6:244–251, with permission.)
Bile is produced by hepatocytes and secreted into biliary canaliculi via canals of Hering, which are trough-like structures bordered by both hepatocytes and cholangiocytes that then drain into bile ducts.
III. Hepatic Blood Supply
The liver receives 25% of the total cardiac output, accounts for 20% of resting oxygen consumption, and together with the splanchnic vascular bed contains 10% to 15% of the total blood volume. The liver’s dual blood supply consists of 75% portal blood (deoxygenated blood) and 25% hepatic arterial blood (oxygenated blood). Although portal blood is deoxygenated, its higher flow results in equivalent oxygen delivery to the hepatic artery. The valveless portal vein acts as a capacitance vessel, while the hepatic artery is a resistance vessel that is dependent on systemic arterial pressure and flow. Most blood enters the hepatic sinusoids from portal venules through inlet sphincters, although branches of hepatic arterioles also terminate in sinusoids near the portal venules (arteriosinus twigs).
 VIDEO 6-1
VIDEO 6-1
Liver Blood Volume
If portal flow decreases, arterial blood supply can be upregulated by vasodilatory molecules such as adenosine and nitric oxide. These mechanisms are not affected by either autonomic innervation or systemic humoral factors. The liver produces a significant volume of lymph through direct exudation from hepatic arterioles and constitutes 25% to 50% of the total lymph flow through the thoracic duct.

Due to its high blood flow (25% of cardiac output) and immense blood filtration capacity, the liver produces up to 50% of all lymph volume flowing in the thoracic duct.
The spongy nature of the liver, combined with the contractile potential of stellate cells, allows this organ to function as an autologous reservoir that can augment blood volume in hypovolemic states and can store blood in hypervolemic states. This latter phenomenon can be observed in conditions of right heart failure (congestive hepatopathy), hypervolemia (renal failure), and iatrogenic overresuscitation.
IV. Hepatic Metabolic, Synthetic, and Excretory Functions
The liver performs a remarkable spectrum of metabolic and excretory functions, ranging from nutritional substance uptake from the portal and systemic circulations, to synthesis of various proteins and bile components, to regulation of circulating nutrients and toxins, to immune host defense (1). Examples of these functions include:
• Bile synthesis and regulation: Bile is composed of various hepatic metabolic products, including cholesterol, phospholipids, bilirubin, and bile salts. Its secretion into the intestinal tract facilitates lipid emulsification, lipid absorption, and excretion of toxins and lipophilic drugs. Impaired bilirubin metabolism or bile secretion (e.g., gallstones) can lead to jaundice and malabsorption of dietary fats (steatorrhea).
• Protein synthesis: Most blood proteins (except antibodies) are synthesized in and secreted by the liver, including albumin, the vitamin K–dependent coagulation factors (II, VII, IX, and X), and the vitamin K–independent factors (V, XI, XII, and XIII and fibrinogen). These can be assessed clinically as indirect indicators of hepatic synthetic function. Amino acid synthesis and breakdown also occur in the liver, the latter by transamination and oxidative deamination, with formation of keto acids, ammonia, and glutamine. Disruption of these synthetic functions can be observed in extreme starvation or liver failure, leading to hypoproteinemia, ascites formation, and bleeding disorders.
• Production of cholesterol and lipoproteins: The liver transforms ingested cholesterol
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








