TABLE 52.1 Arterial Catheter Complications | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
placement (wearing sterile gloves) prevents the contamination of the access site at the outset. In a random sampling, arterial catheter system stopcocks had a contamination rate as high as 38%.19 Occlusive caps or syringes kept on all stopcock ports, as well as irrigating them to clear blood (bacterial culture medium) after any sampling, are appropriate preventive measures.
Catheter placement by cutdown rather than percutaneously
Catheters left in place for longer than 4 days
Catheters left in patients who have experienced sepsis
Glucose-containing flush solution
TABLE 52.2 Guidelines for Care of Arterial Catheters | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
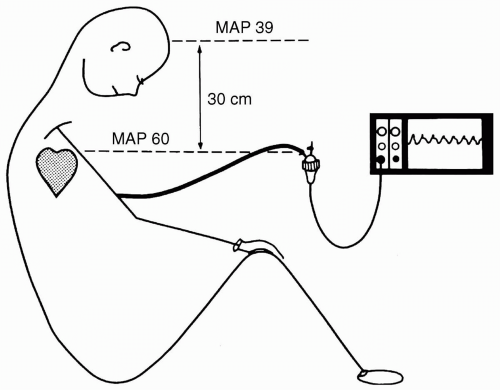
is not kept constant. If, for example, the patient’s bed is raised 30 cm while the transducer is kept mounted on an intravenous (IV) pole, then the height difference between the zero reference (midaxillary line) and the transducer adds the weight of the fluid column in the pressure monitoring tubing (30 cm H2O) to the measured pressure. This raises the apparent pressure by the change in height between transducer and zero reference, that is, 30 cm H2O × 0.7 mm Hg per cm H2O = 21 mm Hg overestimation. The same effect is noted when the transducer is mounted on the head of the bed and the bed is moved into a Trendelenburg position, or when the transducer is patient-mounted to the wrist and the bed is tilted to the side of the transducer. The opposite effect occurs if the patient’s zero reference position is lowered in relation to the transducer.
A three-point calibration to verify that the system is linear
Done in the range of pressures expected to be monitored, for example, arterial pressure 200, 100, and 50 mm Hg
Done using a mercury manometer or equivalent as reference
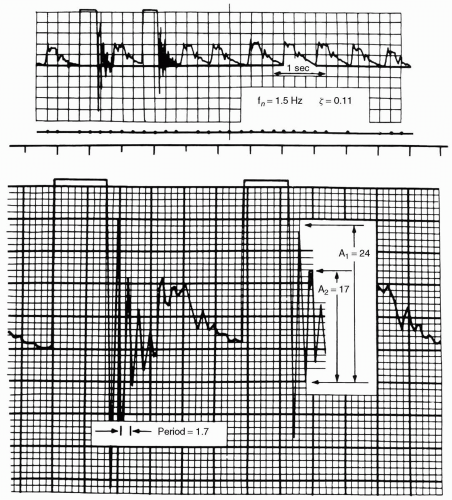
to a system which is ringing (underdamped) and results in higher systolic and lower systolic pressures, a damped system gives lower systolic and higher diastolic pressures. Typically, systolic pressure is affected more than diastolic pressure by both ringing and damping. Mean pressure is the best-preserved and most accurate value in the face of either ringing or damping.
fluid path. Utilizing T-connectors is discouraged because they are compliant and serve as air bubble reservoirs or point of air entrainment during blood sampling if they have a needleless access. It is impossible to predict the relative contributions of each of these factors; therefore, if there is a clinically significant discrepancy between invasive and noninvasive pressures, the flush test provides a simple way to assess the system’s dynamic response characteristics. This, coupled with zero reference, and zero and calibration verification assures the accuracy of the system in use. If the test demonstrates inadequate dynamic response, each of the potential causes should be ruled out. If dynamic response, zero, and gain are correct, consider other causes discussed in the subsequent text.
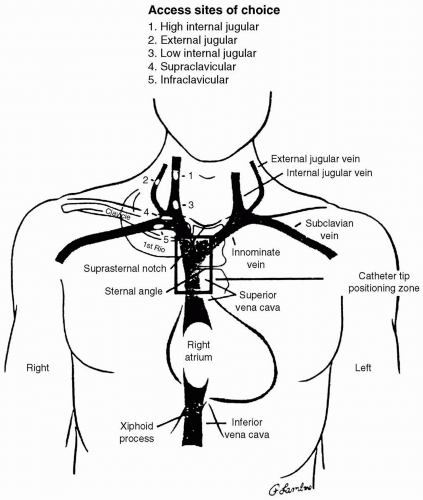
cannulation, occasionally resulting in death.33 Vertebral artery puncture during CVC placement has similarly been associated with fatal stroke.34 Hemothorax is reported following subclavian artery puncture. If CVC placement is required in a patient with a coagulopathy, the subclavian approach is to be avoided. Unlike with carotid artery puncture, it is difficult to apply direct pressure to the subclavian artery or to observe it for hematoma formation. A series of 1,000 IJV placements in patients with coagulopathies resulted in no complications referable to a 7% incidence of carotid artery puncture.32 One patient with a goiter required surgical decompression of a venous bleeding site.32 These authors suggest that IJV cannulation does not result in severe complications in patients with a coagulopathy. Despite these findings, the external jugular route may be preferable in patients with coagulopathy because it avoids the danger of arterial puncture.
TABLE 52.3 Complications due to Central Venous Catheter Placement | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
position is avoided (risk of air embolism), nor is it reliable when CVCs are placed in extremely hypotensive patients, because the central venous pressure (CVP) and arterial pressure may be comparable.
Nitrous oxide diffuses into a pneumothorax much more rapidly than air diffuses out, and therefore the size of the pneumothorax may double in <10 minutes in the presence of 50% N2O.50
Not all pneumothoraces are evident immediately on postplacement chest radiograph.
The use of intraoperative positive pressure ventilation can increase the size of a pneumothorax.
or catheter. Treatment consists of withdrawing the wire or catheter. Mechanically induced arrhythmias resolve upon removal of the stimulus. The lengthy guidewire is most commonly the culprit. A prospective study demonstrated that rigorous control of the depth of guidewire insertion markedly reduced the incidence of arrhythmias during pulmonary artery catheter (PAC) introducer insertion from 58% to 15%.51 An additional benefit was that limiting guidewire insertion depth to <22 cm from the right IJV approach virtually abolished the more hemodynamically severe, ventricular arrhythmias.51