
HYPOTHERMIA: TREATMENT AND THERAPEUTIC USES
There is a clear distinction between hypothermia due to environmental exposure and hypothermia associated with injury. Hypothermia due to exposure can be lethal when it is extreme and persistent, such as that experienced by soldiers during the Napoleonic invasion of Russia; however, with modern medical care, patients without injury have an 80% survival when the depth of hypothermia is between 28°C and 32°C.1 The effect of hypothermia on injured patients, however, is profound. In one large series, no patient with an initial core body temperature of <32°C survived.2 This distinction clearly indicates that a different approach is needed for injured patients compared to medical patients suffering from hypothermia.
The triad of hypothermia, coagulopathy, and acidosis has been widely described as an important risk factor for death among injured patients.3,4 Each component of the triad influences the other components. For instance, hypothermia can lead to worsened coagulopathy, which in turn can lead to acidosis. The presence of hypothermia worsens the physiologic condition of most patients with multisystem trauma. For decades, hypothermia has been treated aggressively in order to optimize outcomes. More recent data suggest there may be a role for induced hypothermia in certain types of injuries. This chapter reviews the physiologic aberrations that are seen with hypothermia and subsequently reviews basic science and clinical studies that guide the current clinical practice of treating injured patients with subnormal core body temperatures.
DEFINITIONS AND MEASUREMENT
There are a variety of definitions of hypothermia. The normal human core body temperature is 37°C; however, there is a normal circadian shift of ±0.5°C-1°C.5,6 Several authors have defined hypothermia as a core body temperature of <35°C, while others have referred to hypothermia as a core body temperature of <36°C.3,7–10 Yet another defined hypothermia as a core body temperature of <37°C.
The traditional classification of hypothermia was developed for patients with environmental exposure rather than patients with multiple injuries.9 The traditional classification of hypothermia labels core body temperatures above 32°C as mild and core body temperatures below 28°C as severe. Profound hypothermia has been defined as core body temperature of 6°C-10°C and ultraprofound hypothermia as temperatures ≤5°C.10 The lowest known temperature among adult survivors with environmental hypothermia is 13.7°C.
The combination of hypothermia and severe injury is associated with increased risk of death and complications. The key threshold associated with increased morbidity and mortality in injured patients appears to be 34°C.2 The risk of mortality is further increased with temperatures below 32°C. As such, a different classification system should be utilized with injured patients. It is most appropriate to consider temperatures of 34°C-36°C as mild, 32°C-34°C as moderate, and <32°C as severe hypothermia. The existence of multiple classification systems can create confusion when interpreting descriptions of the depth of hypothermia. Whenever possible, we have described absolute temperature levels rather than qualitatively labeling the depth of hypothermia.
There are a variety of methods available to evaluate core body temperature (Fig. 53.1). The gold standard for evaluating core body temperature is the pulmonary artery catheter; however, this method is infrequently available during early evaluation and is less commonly utilized in current practice.11 Additionally, in patients with severe hypothermia (<28°C-30°C), this catheter has the potential to induce life-threatening arrhythmias. We recommend the use of bladder or tympanic temperature probes for routine temperature evaluation in acute care surgical patients. Bladder temperatures are measured using a Foley catheter with a thermistor at its tip. Tympanic temperature is readily measurable using current technology; however, many electronic thermometers do not allow measurement of temperature below 34°C. Esophageal temperature monitoring can be used but carries no advantage over the other methods.12 Rectal monitoring is slightly more invasive and is not as responsive to changes in core body temperature. While oral temperature probes are appropriate for healthy patients, these probes are not useful for multiply injured patients or intubated patients. Finally, axillary temperature monitoring is the most inaccurate method and has no real utility in evaluation of hypothermia in acute care surgical patients.3
FIGURE 53.1. Current methods of measuring body temperature. A: Electronic thermometer configured for oral/rectal use. Electronic thermometer for tympanic measurements. C: Foley catheter with temperature-sensitive thermistor. D: Pulmonary artery catheter with temperature-sensitive thermistor.
PREVALENCE AND RISK FACTORS
Hypothermia is common in patients suffering traumatic injury. Trauma patients represent a frequent source of hypothermia.8,9,13 The average temperature of patients in one large trauma center was 35°C.14 Contrary to expectations, there was no seasonal variation, so hypothermia is a consideration regardless of the time of year. Previous reports have estimated that about one-half of seriously injured patients are hypothermic on presentation.13 Patients with prolonged extrications or entrapment are at increased risk, as they often have prolonged environmental exposures. Patients at extremes of age are at increased risk, as their ability to endogenously maintain their core body temperature is reduced. Administration of anesthesia predisposes to hypothermia as these agents inhibit endogenous reflexes that increase core body temperature. Increased injury severity itself is also a risk factor for hypothermia. Submersion is a considerable risk factor, as heat losses are 32 times greater among patients submerged than patients exposed to air.15,16
PHYSIOLOGY
There are physiologic effects of hypothermia on nearly every organ system (Table 53.1). There is increase in metabolic activity at very mild levels of hypothermia (35°C-36°C). Beyond this level, there is a slowing of metabolic activity at the cellular level and quiescence of signs of life at the extremes of hypothermia. In fact, the overall rate of metabolism decreases by 8% for every 1°C reduction in core body temperature.17 Acute care surgeons must understand these physiologic changes in order to optimize treatment of patients who require either induction of hypothermia or institution of warming.
TABLE 53.1
PHYSIOLOGIC CHANGES ASSOCIATED WITH HYPOTHERMIA
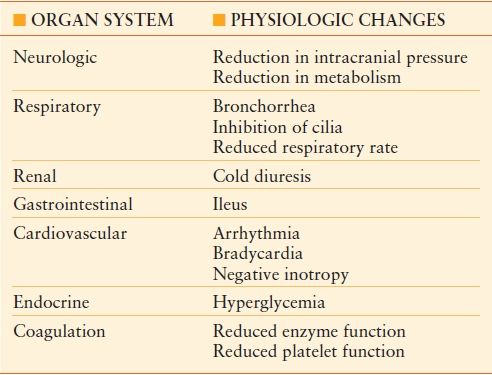
The hypothalamus is responsible for integrating signals from the body that relate to core body temperature.18 The hypothalamus emits efferent signals that result in peripheral vasoconstriction in order to preserve heat to the body core. Shivering is induced with mild hypothermia (35°C-37°C) and can produce up to five times the normal metabolic heat production. Metabolic activity increases in order to generate heat. These mechanisms, however, are impaired as hypothermia deepens to 34°C, and they are also impaired in the multiply injured or intoxicated patient. As a result, injured patients have a reduced ability to endogenously correct hypothermia.
Respiratory system. The respiratory system is stimulated at temperatures above 34°C-35°C. This leads to tachypnea and can result in bronchospasm. As the core body temperature falls below 34°C, the respiratory drive diminishes. There is also an increase in secretions and inhibition of bronchial cilia.8 Upon rewarming, there is often development of pulmonary edema. As core body temperature decreases below 30°C, apnea may result.
Cardiovascular system. Mild hypothermia is associated with cardiac stimulation.8,18 The hypothalamus responds to mild hypothermia by transmitting signals that result in sympathetic activation. This results in tachycardia, peripheral vasoconstriction, and increase in cardiac output. Temperatures below a threshold level (~32°C) result in cardiac depression.9 Negative inotropy is seen with subsequent reductions in cardiac output. Bradycardia ensues, and the myocardium becomes progressively arrhythmogenic.17 Ventricular fibrillation (VF) may occur spontaneously at temperatures below 25°C-28°C.19 Resuscitation from asystole or fibrillation can be difficult, if not impossible, until the patient is warmed. These arrhythmias are often resistant to chemical or electrical cardioversion until the core body temperature is raised above 28°C. Additionally, there is a reduced response to catecholamines with appreciable degrees of hypothermia.17,20
Neurologic. There is a temperature-dependent reduction in consciousness associated with progressive hypothermia.21 Confusion can be expected at mild degrees of hypothermia. This will eventually give way to a comatose state at temperatures <28°C-30°C. There is a reduction in cerebral blood flow and cerebral metabolism, which leads to a reduction in intracranial pressure. Motor reflexes become diminished and ultimately become absent.9 These cerebral effects have led to a great deal of interest in utilizing hypothermia as a therapeutic entity for conditions associated with neurologic compromise. This is described in greater detail later in the chapter.
Renal/electrolytes. “Cold diuresis” is the most obvious effect of hypothermia on the renal system.17,22 This is seen at mild and moderate degrees of hypothermia (>32°C). This is due to increased renal blood flow and to alterations in tubular membranes. The pH measured by arterial blood gas analysis will be artificially low if the blood is warmed before the analysis is conducted. Hyperglycemia can occur as a result of reduced insulin sensitivity and decreased pancreatic secretion of insulin. Electrolytes, including potassium, phosphorus, and magnesium, can become depleted.23
Gastrointestinal. Bowel function in the presence of hypothermia is reduced.17 If a nasogastric tube is inserted for an ileus in a hypothermic patient, extreme caution should be taken. Gastric intubation, along with other methods of patient manipulation, can lead to VF in patients with a core body temperature <28°C.9
Coagulation. Coagulation disorders represent the most important physiologic disturbance concerning hypothermic acute care surgical patients. These disorders are also the most extensively studied issues in injured patients.–2435 Hypothermia leads to coagulopathy. Ongoing hemorrhage from coagulopathy leads to further hypothermia and acidosis. This “bloody vicious cycle” first reported by Kashuk et al.36 has been confirmed in multiple subsequent studies. Warming hypothermic patients is a critical component to arresting this cycle.
The effect of hypothermia on standard laboratory values of prothrombin time/activated partial thromboplastin time/International Normalized Ratio (PT/PTT/INR) has been extensively reported. Cosgriff et al.29 prospectively evaluated patients who required a massive transfusion, which was defined as transfusion of at least 10 units of blood. Coagulopathy was defined as a PT greater than two times the normal value and was present in 47% of the patients. In multivariate analysis, hypothermia was a significant risk factor for coagulopathy, with an odds ratio of 8.7.
These clinical results have been confirmed by a number of laboratory studies. Reed et al.31 submerged anesthetized rats in a water bath to achieve a core body temperature of 25°C-37°C. They observed a prolongation of the PTT and the PT when the blood was not warmed prior to analysis. When they warmed the blood ex vivo , the prolongation of the coagulation parameters was no longer seen. This suggests that laboratory analysis that is conducted after warming the blood ex vivo is likely to underestimate the degree of coagulopathy.
Rohrer and Natale32 evaluated pooled human plasma that was known to have normal levels of clotting factors. They performed coagulation studies on the blood at temperatures ranging from 28°C-41°C. The PT in the normothermic samples was 11.8 seconds and increased to 16.6 seconds in the samples cooled to 28°C-34°C. The PTT was 36 seconds in the normothermic samples and increased to 57 seconds in the hypothermic samples. These results were confirmed clinically by Watts et al.33 They evaluated 112 trauma patients with an injury severity score ≥9 points. They evaluated the core body temperature and performed coagulation studies. They found that 34°C was the threshold point, below which coagulation enzyme activity decreased.
Another study compared the effect of hypothermia to various clotting factor deficiencies.30 Standard plasma was evaluated at temperatures between 25°C and 37°C, and plasma with specific clotting factor deficiencies was evaluated for comparative purposes. They found that plasma with normal factors at temperatures <33°C was functionally similar to normothermic plasma with factor deficiencies.
Hypothermia in an injured patient is often seen in combination with shock. In an animal model, hypothermia and shock were additive in their effects on coagulopathy.28 In a human series, coagulopathy was also more common when hypothermia was paired with an increased injury severity and shock.29
Platelet function is also reduced in hypothermic patients. Valeri et al.35 noted that a reduction in core body temperature in baboons was associated with a prolonged bleeding time. A similar study was conducted in healthy human volunteers. The bleeding time of hypothermic skin was significantly prolonged. This appeared to be mediated by a down regulation of P-selectin and thromboxane B2.34
Whereas the most common and standard method of evaluating coagulation abnormalities involves measuring the PT/PTT/INR, Martini et al.25 reported that thromboelastography (TEG) is a more sensitive analysis in determining hypothermic coagulopathy. This point-of-care test evaluates the entire coagulation system in whole blood. TEG may be more accurate than are standard tests for evaluation of multiple aspects of the coagulation cascade and in directing therapy. TEG is becoming more available but has not currently been broadly adopted.
Two reports have evaluated pharmacologic manipulation of hypothermic coagulopathy.26,27 Given that factor levels are normal, treatment with fresh frozen plasma would not be expected to reverse the coagulopathy. The administration of recombinant factor VIIa (rFVIIa), however, improved coagulation parameters of plasma that was artificially cooled. In a model of hypothermic/hemorrhagic shock, the administration of rFVIIa reduced overall blood loss. This suggests that rFVIIa can be used as an adjunct to warming when ongoing hemorrhage is severe.
In summary, there is a clear effect of hypothermia on the clotting cascade. This coagulopathy is caused by an alteration in platelet function and enzymatic activity rather than an alteration in the quantity of clotting factors. This effect is temperature-dependent, with deeper levels of hypothermia being associated with more substantial coagulopathy. Furthermore, common laboratory tests do not reflect the true degree of coagulopathy if the blood is warmed ex vivo prior to performing the test, as is commonly performed by most labs. The treatment of hypothermic coagulopathy is rapid warming rather than administration of blood products or supplements.
CLINICAL CONSIDERATIONS
Given the many physiologic effects of hypothermia on mammals, it is important to understand the clinical ramifications of reduced core body temperatures. There are certain responses to hypothermia that may be beneficial to acute care surgical patients, such as a reduction in metabolic rate and cerebral protection. There are other responses to hypothermia that are clearly harmful, such as coagulopathy and myocardial irritability. There is considerable of literature evaluating the effect of hypothermia on injured patients. The majority of the experimental studies have reported a beneficial effect of hypothermia on outcomes in injured patients, whereas most of the human studies have reported on a detrimental effect of hypothermia.
Numerous animal models have touted the benefit of hypothermia in injured patients.–3743 Wu et al.40 evaluated a model of hemorrhagic shock in pigs by creating a splenic laceration. Animals were subsequently cooled to 34°C or maintained at normothermia. Survival was greater in the hypothermic animals, and increased bleeding was not seen in the hypothermic animals. In another similar study using a model of prolonged hypotensive resuscitation, George et al.44 demonstrated improved survival in hypothermic animals with associated decreases in resuscitation fluid requirements, organ injury, and lactate concentrations. Takasu et al.41 reported similar findings in a rat model. Animals subjected to hemorrhagic shock that were cooled to 32°C or 34°C had a greater 72-hour survival. Another study also reported the benefits of hypothermia during hemorrhagic shock but also noted that these benefits were not realized with localized gut cooling.38 In a rat model of hemorrhagic shock, Lee et al.43 reported that induced hypothermia was more beneficial than spontaneous hypothermia. Another report confirmed that spontaneous hypothermia did not result in increased long-term survival.45
Specific organ function has also been evaluated in animal models. Severe hypothermia (28°C) in conjunction with hemorrhagic shock was associated with ventricular irritability.46 The effect of shock on liver adenosine triphosphate (ATP) concentrations was reported by Johannigman et al.47 In a rat model of hemorrhagic shock, ATP fell steadily with warm shock but not hypothermic shock.
The process of warming has also been evaluated in animal models. In rat and mouse models of hemorrhagic shock, there was a considerable inflammatory response associated with the warming phase.48,49 Warming improved cardiac and hepatic function in a rat model of hemorrhage.50 Survival was greatest among pigs that underwent a “medium” rate of warming compared with slow and fast rates of warming.42
In contrast to the animal data, much of the human data suggest that hypothermia in injured patients is harmful.2,51–54 A sentinel study was reported by Jurkovich et al. in 1987. They evaluated the effect of hypothermia on a group of patients with major trauma. Mortality progressively rose with reductions in core body temperature.2 No patients in this series survived if their core body temperature was below 32°C. Wang et al.52 conducted a multivariate analysis of risk factors of mortality after injury and noted an odds ratio for death of 3.03 associated with hypothermia. Shafi et al. attempted to discern if hypothermia was a marker of complications or a true risk factor for death in injured patients. They evaluated nearly 40,000 patients from the National Trauma Data Bank (NTDB) and performed a multivariate regression analysis.53 They found that hypothermia (≤35°C), independent of other factors, was associated with increased mortality. Another large review of the NTDB noted that mortality began to increase at temperatures below 36°C.55 All of these studies were retrospective analyses. In one prospective analysis, approximately 300 seriously injured trauma patients were studied, and effects of hypothermia on outcome were evaluated.56 Hypothermia within the first six hours of arrival was not independently associated with mortality but did predict development of multiple organ failure. In the one randomized prospective study published to date, Gentilello et al.51 randomized patients to rapid rewarming with continuous arteriovenous rewarming (CAVR) versus standard rewarming. Patients in the CAVR group experienced faster rates of warming. Early mortality, as well as 24-hour fluid requirements, were improved in patients randomized to CAVR. However, this benefit did not result in improved survival to hospital discharge. These studies together suggest that hypothermia is harmful in injured patients.
The distinction between elective hypothermia and trauma-related hypothermia was reported by Seekamp et al. They measured plasma ATP concentrations in patients undergoing elective hypothermia during coronary artery bypass surgery and in hypothermic trauma patients.57 They noted that there was a preservation of ATP levels in elective hypothermia but decreased level of ATP in hypothermic injured patients. This suggests that hypothermia in injured patients is due to energy exhaustion rather than being a method of energy preservation.
Several reports have noted the effect of hypothermia on injured patients who require operative intervention.–5860 An increase in mortality was noted among hypothermic patients who required a laparotomy. Blood loss was also related to the depth of hypothermia. Notably, the degree of hypothermia was more important than the abdominal injury score in determining the amount of intraoperative blood loss.
The distinction between human and animal studies likely relates to the type of studies that have been performed. Nearly all of the human studies have been retrospective analyses. Given the uniformity of the results, surgeons have been unwilling to randomize multiply injured patients to hypothermia or normothermia in a prospective study. The animal studies have generally not involved multi-system trauma. It is unclear if single-organ models of hemorrhagic shock in animals apply to the injured patients that acute care surgeons regularly encounter. Additionally, animal models generally involve active cooling of an otherwise normothermic animal. Human studies evaluated patients who were hypothermic on presentation. Finally, animal models induce hypothermia shortly after the time of injury or before the injury. Given that there are inherent delays in transport of human trauma, it is not clear that these models are applicable to clinical settings.
Although the evidence is incomplete, we recommend warming multiply injured patients to normothermic levels. There is interesting experimental evidence suggesting that hypothermia may be beneficial during the course of injury, especially when associated with prolonged periods of hypotension,44 but the application of this evidence is currently unclear. The bulk of the human evidence demonstrates that hypothermia is harmful to injured patients. Until the animal models are replicated in human studies, acute care surgeons should consider hypothermia to be a complicating factor in the management of their patients.
REWARMING
There are a number of methods to rewarm injured patients. These methods should be instituted early in the treatment of injured patients, as it is far easier to prevent further hypothermia than it is to treat hypothermia. An injured patient’s metabolic rate may be sufficient to prevent further heat loss, but it is insufficient to produce overall warming.61 Accordingly, the majority of severely injured patients will require adjunctive warming measures.
An average adult with a temperature of 32°C has a massive heat deficit of 300 kCal.61 Oxygen consumption would have to increase dramatically to overcome this heat deficit, and severely injured patients generally do not have the physiologic reserve to respond to this challenge. The human body has a high specific heat (amount of energy required to raise 1 kg of material by 1°C). This, along with ongoing heat losses, makes warming injured patients challenging.
Methods of Rewarming
Methods of rewarming can be broadly categorized as passive or active (Table 53.2). Passive methods consist of blankets and a warm environment. Active methods can be characterized as either invasive or noninvasive. Noninvasive methods include forced air rewarming, circulating water blankets, radiant heat lamps, and resistive heating blankets. Invasive methods include tracheal insufflation with warmed gas, cavity lavage, cardiopulmonary bypass, and arteriovenous rewarming.
TABLE 53.2
METHODS OF WARMING
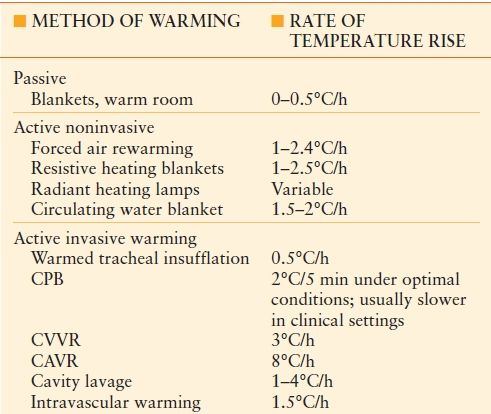
CPB, cardiopulmonary bypass; CVVR, continuous veno-venous rewarming; CAVR, continuous arteriovenous rewarming.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









