Hyperthermia
Barbara W. Brandom
CASE SUMMARIES
CASE #1: A child presented for computerized tomographic-guided biopsy of nodules on the diaphragm, placement of a Broviac catheter, and lumbar puncture with general anesthesia. In the last 2 weeks, abdominal girth had been increasing. Ascites and pleural effusions were noted. Oral temperature was 39.5°C. Is this hyperthermia? How should it be treated?
CASE #2: A healthy adolescent underwent elective orthopedic surgery during inhalation anesthesia. A laryngeal mask airway was used. Axillary temperature was <36°C, so the lower body was warmed with 43°C forced air. When laryngospasm was perceived, an endotracheal tube and an esophageal temperature probe were placed. Esophageal temperature was >41°C. Is this hyperthermia? How should it be treated?
CASE #3: A middle-aged woman with a body mass index >40 kg per m2 underwent laparoscopic Roux-en-Y gastric bypass. Preoperative laboratory evaluation was normal. Her course in the operating room and postanesthesia care unit (PACU) was unremarkable. Seven hours after the operation, the patient’s heart rate was 120 bpm, and her axillary temperature was 38.2°C. Blood pressure was 140/60 mm Hg. A Foley catheter relieved urinary retention. She stated that patient-controlled analgesia was providing good pain relief. Hematologic laboratory tests and serum electrolytes were normal. Tachycardia and fever persisted overnight despite the administration of 2 L of isotonic crystalloid intravenously.
The anesthesiologist may encounter a patient with elevated core temperature preoperatively, intraoperatively, or after surgery and anesthesia have been completed. This chapter presents the basic elements of thermoregulation that are relevant to maintaining body temperature greater than normal. Several situations that produce more heat than expected or prevent dissipation of heat will be presented. The life-threatening consequences of critical temperature will be reviewed, and interventions that can be used to decrease body temperature will be described.
What Is the Relationship between Hyperthermia and Thermoregulation?
Hyperthermia is an elevation of core body temperature above 38°C for any reason. Normally, core temperature is maintained in a narrow range, ±0.2°C, by thermoregulatory reflexes,1 although an interperson variability of 2°C in normal core temperature2 and diurnal variability of approximately 1°C can occur.3,4
▪ HEAT PRODUCTION
Heat production is divided into two processes: Obligatory and facultative.5 Obligatory thermogenesis is a result of the metabolic processes necessary to sustain life. Facultative thermogenesis is a rapidly inducible process driven by the hypothalamus and sympathetic nervous system in response to cold or excess food intake.6 Facultative thermogenesis can increase the thermoregulatory setpoint by 0.3°C.7
▪ HEAT LOSS
Evaporation
The maintenance of thermal steady state requires the dissipation of heat produced by the metabolism to the relatively cool environment. Almost all of this heat moves through the skin, the rest being lost through the respiratory tract. When core temperature rises above a reproducible threshold value, sweating and vasodilation occur. Skin blood flow can increase by 8 L per minute.8 In a dry environment with moving air, evaporation of sweat can dissipate heat at 10 times the basal metabolic rate.9 Significant amounts of salt and up to 2 L per hour of water can be lost in sweat.10,11 At rest, without sweating, evaporative heat loss is only approximately 5% of the basal metabolic rate.
Conduction
Heat is also lost by conduction, convection, and radiation as a linear function of the difference between skin and ambient temperatures. Conduction is the direct transfer of heat from one surface to an adjacent surface. Insulation between two surfaces will reduce conduction.
Convection
Convection is loss of heat to moving air, in proportion to the square root of the velocity of the air. With a 15 to 20 mph wind velocity, heat loss by convection is at a maximum.
Radiation
Radiation is the transfer of heat from one body to another through photons. Therefore, radiation does not depend on the temperature of the surrounding air.12 When environmental temperature is less than core temperature, vasodilation allows core temperature to decrease, because heat is lost to the environment through conduction and convection. When the patient is not insulated from the cooler environment by materials such as heavy clothes, heat loss by conduction, convection, evaporation, and radiation is greater.
Why Does Hyperthermia Occur?
Hyperthermia can be passive or active. It is the result of one or more of the following factors: Decreased heat loss to the environment, constraint of heat to the core thermal compartment, increased metabolic production of heat,13 or excessive delivery of exogenous heat. With current forced-air warming devices, it is possible to deliver excessive amounts of heat to a patient during the administration of anesthesia. Decreased environmental heat loss occurs when the patient’s insulation is effective and prevents heat loss by conduction, convection, radiation, or evaporation. Evaporative environmental heat loss is reduced when humidity is high.
During anesthesia, the threshold temperature at which sweating begins is increased. For example, exposure to 1.2% isoflurane increases the sweating threshold from an average of 36.6°C to 38.1°C in men and from 37.1°C to 38.3°C in women14 (see Fig. 46.1). However, the maximum sweating intensity and the gain of this response (the increase in sweat production for each unit increase in temperature) are not altered by an increased anesthetic dose. If the patient cannot produce sweat, heat loss by evaporation depends on the application of exogenous liquid (water or alcohol) to the skin. Heat loss to the environment will not occur by conduction or convection when environmental temperatures are greater than body temperature. Therefore, in a hot and humid environment, otherwise normal anesthetized patients can easily become hyperthermic.
▪ TEMPERATURE GRADIENTS
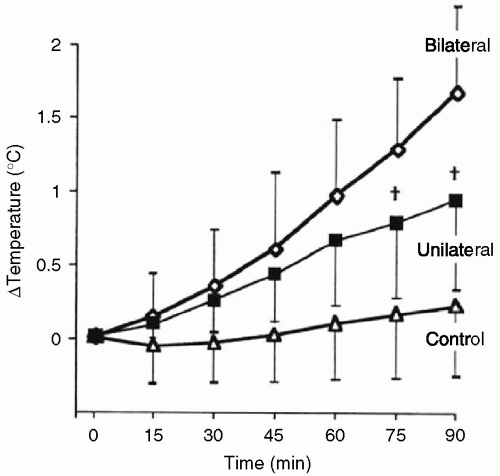
Under normal physiologic conditions in the absence of anesthesia, a gradient in body temperature of 2°C to 4°C is present from the body core to the extremities. Skin temperature is 5°C to 9°C lower than core body temperature measured at the forehead,15 pulmonary artery, esophagus, rectum, or bladder. This heat gradient normally is maintained by vasoconstriction that is controlled by sympathetic reflexes. Heat will be constrained to the core compartment with increased vasoconstriction. This situation may result from low blood volume, low cardiac output,16 or some other source of elevated plasma catecholamines. A 10% decrease in plasma volume is sufficient to compromise convective thermoregulation.17 Retention of heat by the core compartment contributes to the elevated temperature that is one of the signs of untreated pheochromocytoma and to fever after cardiopulmonary bypass in the presence of low cardiac output. Mechanical obstruction to regional blood flow that prevents heat dissipation also is seen. Leg tourniquets have increased core heat sufficient to raise temperature more than 1°C in an anesthetized child18 (see Fig. 46.2).
How Is Fever Produced?
Metabolism is usually reduced approximately 15% by general anesthesia. However, many factors can cause increased metabolism and increased heat production. The most frequent of these is fever, and hyperthermia is most often caused by fever. However, it is important to differentiate between fever and hyperthermia. Fever is defined by a core temperature over 38.5°C because of pyrogens.
▪ THE ROLE OF PYROGENS
Many tissue injuries produce proteins and protein fragments. These and bacterial lipopolysaccharide toxins may
be called exogenous pyrogens.19 These exogenous pyrogens stimulate the release of endogenous pyrogens, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) from macrophages and monocytes. Endogenous pyrogens, in turn, promote the release of prostaglandin (PG) E2 in the preoptic area of the hypothalamus where temperature is regulated.20
be called exogenous pyrogens.19 These exogenous pyrogens stimulate the release of endogenous pyrogens, including interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) from macrophages and monocytes. Endogenous pyrogens, in turn, promote the release of prostaglandin (PG) E2 in the preoptic area of the hypothalamus where temperature is regulated.20
Normal thermoregulatory responses and an elevated set-point are seen with fever. For example, the normal range of core temperature—in which there is no autonomic response that could produce gain or loss of heat—is 0.2°C to 0.3°C. If core temperature is below the lower limit of this interthreshold range, vasoconstriction, nonshivering thermogenesis, and shivering occur. When the interthreshold range is exceeded, sweating and then vasodilation follow. Endogenous pyrogens such as ILs 1, 2, and 6, TNF, and interferon-α increase the core temperature set-point in the middle of the interthreshold range. As a result, body temperature is maintained above 37.2°C and even above 38°C. Such temperature elevation may stimulate immune function, thereby aiding recovery from infection.21 The common finding of preoperative fever in an infected patient does not mean that normothermia should be the goal of an anesthetic administered to facilitate surgical treatment. Shivering in a patient with elevated core temperature is a sign that pyrogens have raised the temperature set-point, and normal thermoregulation is acting to maintain the elevated temperature. Fever may be decreased following the administration of drugs that inhibit PGs. Fever will not respond to external cooling measures without the inhibition of PGs, and they rarely produce temperatures above 41.1°C.
If fever is present preoperatively, temperature may decrease during surgery because the thresholds for vasoconstriction, shivering, and nonshivering thermogenesis are reduced 3°C to 5°C by general anesthesia. The patient may begin anesthesia with a core temperature of 39°C and leave the operating room with core temperature of 36°C or less, because during anesthesia neither vasoconstriction nor an increase in thermogenesis occurred. Furthermore, during anesthesia, internal redistribution of heat from the core to the periphery of the body occurs,22 allowing more heat to be lost to the cold environment.
One study was published of the effects of IL-2 during 0.6 minimum alveolar concentration of desflurane anesthesia. The interthreshold range in temperature between sweating and vasoconstriction was 0.4°C at baseline and after exposure to IL-2, but widened to an average of 1.9°C during exposure to low dose desflurane.23 Such anesthetic-induced impairment of thermoregulation may contribute to temperature elevation in the patient with preoperative fever during recovery from anesthesia. In the hour after anesthetic administration is terminated, if endogenous pyrogens persist, vasoconstriction and shivering will boost core temperature. Higher levels than were present preoperatively may result as the inhibitory effects of anesthetics and intravenous analgesics on thermoregulation dissipate.
▪ POSTOPERATIVE FEVER
Even when no infection is present, postoperative fever is very common. After major noncardiac surgery in adults, the median temperature was 38°C, and 25% of patients had temperature >38.5°C 11 hours after surgery.24 This temperature elevation was associated with increased IL-6. Following cardiopulmonary bypass, IL-6 concentration increases, and fever of 38°C to 39°C is common.25 Major intracranial neurosurgical procedures may be followed by fever over 38.5°C in more than 80% of children, although meningitis is present in only approximately 10%.26 During the rehabilitation phase following brain injury, fever is much less common. In the absence of infection, temperature was less than, 38.2°C, and fever occurred only in those patients with traumatic brain injury or aneurysmal subarachnoid hemorrhage.27
Fever also is common after routine tonsillectomy,28 orthopedic surgery,29 and gynecologic surgery.30 Noninfectious processes are associated with 50% to more than 90% of fevers after gynecologic surgery.31 Years ago, it was recognized that a temperature elevation >38°C occurs in 25% to 30% of children during the first 3 postoperative days. Clinical evaluation was more useful than laboratory tests in determining the cause. Fever was associated with surgery longer than 2-hours duration, intraoperative blood transfusion, preexisting infection, and the administration of preoperative antibiotics. In <2% of these children, sepsis was the reason for fever.32 These fevers are not complications of anesthesia as such; they are the result of pyrogens released by the surgical procedure.
Rarely, postoperative fever is caused by an endocrine abnormality, such as hyperthyroidism,33 which
was not active preoperatively. Case reports of malignant hyperthermia (MH) susceptibility, with or without rhabdomyolysis, or Duchenne muscular dystrophy with exacerbated rhabdomyolysis, presenting with postoperative fever have been published. However, a series of cases selected for evaluation of postoperative fever rarely finds a muscular cause for the temperature elevation.
was not active preoperatively. Case reports of malignant hyperthermia (MH) susceptibility, with or without rhabdomyolysis, or Duchenne muscular dystrophy with exacerbated rhabdomyolysis, presenting with postoperative fever have been published. However, a series of cases selected for evaluation of postoperative fever rarely finds a muscular cause for the temperature elevation.
Paradoxical hyperthermia on exposure to cold has been observed experimentally in cold-adapted rats34; these animals have an increased metabolic rate. A central injection of PGE1 also increased core temperature by 1.9°C in these animals in contrast to a 0.9°C increase in animals made hypermetabolic to a similar degree with thyroxine. Whether similar mechanisms are active in humans is unknown.
▪ DRUGS THAT PRODUCE HYPERTHERMIA
Fevers caused by adverse reactions to drugs that are administered during anesthesia are anesthetic complications. Antibiotics, antihistamines, and barbiturates are among those that have been associated with such drug-induced fever. In some cases, these are allergic reactions. Diphenhydramine, a commonly administered antihistamine, has significant anticholinergic effects which contribute to hyperthermia primarily by decreasing sweat formation.35 Fever can be present in cases of salicylate toxicity.36 Postoperative hyperthermia may follow the administration of ketamine.37
A different form of drug toxicity can produce hyperthermia. In some situations, the factors that produce elevation of the thermoregulatory set-point are complex and not initiated by pyrogens. For example, diatrizoate, an ionic contrast dye used for myelography, affects dopaminergic neurotransmission and produces increased motor activity and thermoregulatory set-point elevation.38 An elevated temperature after the spread of this dye into the cerebrospinal fluid is a result of increased metabolic heat from increased muscle activity and set-point elevation due to dopaminergic stimulation. However, dopaminergic drugs can also produce hypothermia.39 The ascending tonic-clonic syndrome includes hyperthermia and has been noted after cerebrospinal fluid introduction of diatrizoate and two other similar compounds, metrizamide and metrizoate. It is frequently fatal.40
Similarly, 3, 4-methylenedioxymethamphetamine (MDMA, aka ecstasy) alters serotonin,41 dopamine,42 and norepinephrine43 in the hypothalamus, all of which have an effect on thermoregulation. An early effect of MDMA is to release neuronal serotonin which upregulates dopamine biosynthesis and release by activation of serotonin 2A postsynaptic receptors. The subsequent activation of D1 receptors plays an essential role in the hyperthermic response to MDMA.44 MDMA can reset thermoregulation in the hypothalamus, as well as produce effects in the rest of the body that increase production and reduce elimination of heat. The administration of MDMA is followed by a very marked elevation of plasma norepinephrine, which produces vasoconstriction and impedes heat loss, but also stimulates both α1 – and β3-adrenoreceptors. The activation of β3-adrenoreceptors stimulates cyclic adenosine monophosphate (cAMP)-dependent liberation of intracellular free fatty acids. There are proteins, known as uncoupling proteins, which function as catalysts for the movement of anionic portions of fatty acids across mitochondrial membranes. In the presence of uncoupling proteins, increased intracellular free fatty acid concentrations result in the decreased generation of adenosine triphosphate (ATP) and increased thermogenesis.45 Therefore, uncoupling proteins regulate the balance between ATP production and thermogenesis in muscle and brown adipose tissue46,47 (see Fig. 46.3). Activation of α1-adrenoreceptors strongly potentiates the thermogenic effect of β3-adrenoreceptor activation.48 Perhaps MDMA will very rarely be found in patients presenting for anesthesia, but other phenethylamine sympathomimetics may well be seen. Amphetamine, methamphetamine, and structurally different stimulants such as cocaine can have similar uncoupling of thermogenesis and ATP production in muscle and other cells, as can disease states in which endogenous catecholamines are elevated.
Recognition of the pharmacologic mechanisms that can produce hyperthermia is important. Epinephrine and dobutamine do not activate the β3- adrenoreceptor and do not have thermogenic effects. Conversely, thyroid function has a significant effect on sympathomimetic-mediated thermogenesis. Expression of uncoupling proteins in different tissues is regulated through transcription by thyroid hormone and dietary fatty acids.49
Serotonin and Neuroleptic Malignant Syndromes
Other hyperthermic syndromes in which nonadrenergic stimulation of the central nervous system may play a part include the serotonin syndrome and the neuroleptic malignant syndrome (NMS). The serotonin syndrome includes cognitive, autonomic, and neuromuscular dysfunction, as well as hyperthermia, clonus, and muscle rigidity in a patient taking one or more of the serotoninergic drugs.50 Monoamine oxidase inhibitors, tricyclic antidepressants, serotonin reuptake inhibitors, and lithium have been associated with the serotonin syndrome, as have the analgesics meperidine, dextromethorphan, and tramadol.51 Recently, a few cases of serotonin syndrome have been reported after the addition of oxycodone to the treatment of patients taking other drugs that can alter the serotoninergic and dopaminergic systems.52 Perhaps increased dopamine and glutamate concentrations in the hypothalamus contribute to the pathophysiology of the serotonin syndrome.53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree