Key Concepts
 The hepatic artery supplies 45% to 50% of the liver’s oxygen requirements, and the portal vein supplies the remaining 50% to 55%.
The hepatic artery supplies 45% to 50% of the liver’s oxygen requirements, and the portal vein supplies the remaining 50% to 55%.
 All coagulation factors, with the exception of factor VIII and von Willebrand factor, are produced by the liver. Vitamin K is a necessary cofactor in the synthesis of prothrombin (factor II) and factors VII, IX, and X.
All coagulation factors, with the exception of factor VIII and von Willebrand factor, are produced by the liver. Vitamin K is a necessary cofactor in the synthesis of prothrombin (factor II) and factors VII, IX, and X.
 Many “liver function” tests, such as serum transaminase measurements, reflect hepatocellular integrity more than hepatic function. Liver tests that measure hepatic synthetic function include serum albumin, prothrombin time (PT, or international normalized ratio), cholesterol, and pseudocholinesterase.
Many “liver function” tests, such as serum transaminase measurements, reflect hepatocellular integrity more than hepatic function. Liver tests that measure hepatic synthetic function include serum albumin, prothrombin time (PT, or international normalized ratio), cholesterol, and pseudocholinesterase.
 Albumin values less than 2.5 g/dL are generally indicative of chronic liver disease, acute stress, or severe malnutrition. Increased losses of albumin in the urine (nephrotic syndrome) or the gastrointestinal tract (protein-losing enteropathy) can also produce hypoalbuminemia.
Albumin values less than 2.5 g/dL are generally indicative of chronic liver disease, acute stress, or severe malnutrition. Increased losses of albumin in the urine (nephrotic syndrome) or the gastrointestinal tract (protein-losing enteropathy) can also produce hypoalbuminemia.
 The PT, which is normally 11-14 sec, depending on the control value, measures the activity of fibrinogen, prothrombin, and factors V, VII, and X.
The PT, which is normally 11-14 sec, depending on the control value, measures the activity of fibrinogen, prothrombin, and factors V, VII, and X.
 The neuroendocrine stress response to surgery and trauma is characterized by elevated circulating levels of catecholamines, glucagon, and cortisol. Mobilization of carbohydrate stores and proteins results in hyperglycemia and a negative nitrogen balance (catabolism), respectively.
The neuroendocrine stress response to surgery and trauma is characterized by elevated circulating levels of catecholamines, glucagon, and cortisol. Mobilization of carbohydrate stores and proteins results in hyperglycemia and a negative nitrogen balance (catabolism), respectively.
 All opioids can potentially cause spasm of the sphincter of Oddi and increase biliary pressure.
All opioids can potentially cause spasm of the sphincter of Oddi and increase biliary pressure.
 When the results of liver tests are elevated postoperatively, the usual cause is underlying liver disease or the surgical procedure itself.
When the results of liver tests are elevated postoperatively, the usual cause is underlying liver disease or the surgical procedure itself.
Functional Anatomy
The liver is the heaviest organ in the body, weighing approximately 1500 g in adults. It is separated by the falciform ligament into right and left anatomic lobes; the larger right lobe has two additional smaller lobes at its posterior-inferior surface, the caudate and quadrate lobes. In contrast, surgical anatomy divides the liver based on its blood supply. Thus, the right and left surgical lobes are defined by the point of bifurcation of the hepatic artery and portal vein (porta hepatis); the falciform ligament therefore divides the left surgical lobe into medial and lateral segments. Surgical anatomy defines a total of eight segments.
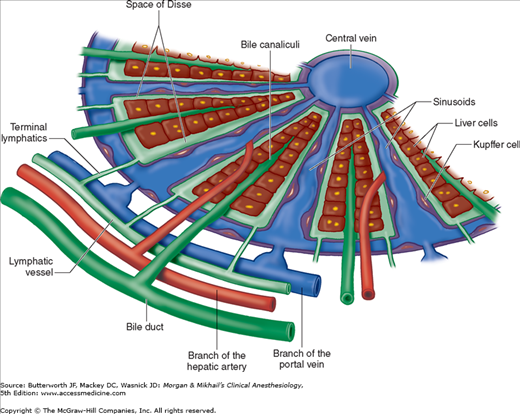
The liver is made up of 50,000-100,000 discrete anatomic units called lobules. Each lobule is composed of plates of hepatocytes arranged cylindrically around a centrilobular vein (Figure 32-1). Four to five portal tracts, composed of hepatic arterioles, portal venules, bile canaliculi, lymphatics, and nerves, surround each lobule.
In contrast to a lobule, an acinus, the functional unit of the liver, is defined by a portal tract in the middle and centrilobular veins at the periphery. Cells closest to the portal tract (zone 1) are well oxygenated; those closest to centrilobular veins (zone 3) receive the least oxygen and are most susceptible to injury.
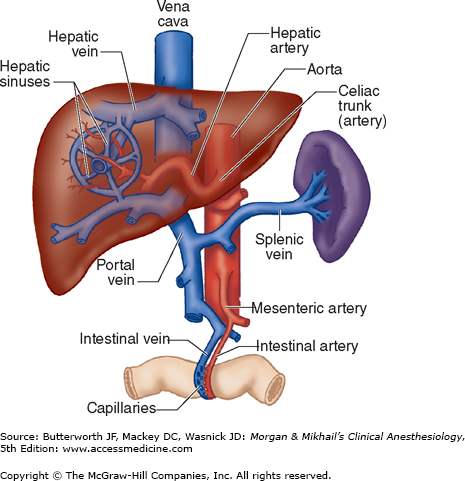
Blood from hepatic arterioles and portal venules comingle in the sinusoidal channels, which lie between the cellular plates and serve as capillaries. These channels are lined by endothelial cells and by macrophages known as Kupffer cells. The Kupffer cells remove bacteria endotoxins, viruses, proteins and particulate matter from the blood. The space of Disse lies between the sinusoidal capillaries and the hepatocytes. Venous drainage from the central veins of hepatic lobules coalesces to form the hepatic veins (right, middle, and left), which empty into the inferior vena cava (Figure 32-2). The caudate lobe is usually drained by its own set of veins.
Bile canaliculi originate between hepatocytes within each plate and join to form bile ducts. An extensive system of lymphatic channels also forms within the plates and is in direct communication with the space of Disse.
The liver is supplied by sympathetic nerve fibers (T6-T11), parasympathetic fibers (right and left vagus), and fibers from the right phrenic nerve. Some autonomic fibers synapse first in the celiac plexus, whereas others reach the liver directly via splanchnic nerves and vagal branches before forming the hepatic plexus. The majority of sensory afferent fibers travel with sympathetic fibers.
Normal hepatic blood flow is 25% to 30% of the cardiac output and is provided by the hepatic artery and portal vein.  The hepatic artery supplies about 45% to 50% of the liver’s oxygen requirements, and the portal vein supplies the remaining 50% to 55% (Figure 32-2). Hepatic arterial flow seems to be dependent on metabolic demand (autoregulation), whereas flow through the portal vein is dependent on blood flow to the gastrointestinal tract and the spleen. A reciprocal, though somewhat limited, mechanism exists, such that a decrease in either hepatic arterial or portal venous flow results in a compensatory increase in the other.
The hepatic artery supplies about 45% to 50% of the liver’s oxygen requirements, and the portal vein supplies the remaining 50% to 55% (Figure 32-2). Hepatic arterial flow seems to be dependent on metabolic demand (autoregulation), whereas flow through the portal vein is dependent on blood flow to the gastrointestinal tract and the spleen. A reciprocal, though somewhat limited, mechanism exists, such that a decrease in either hepatic arterial or portal venous flow results in a compensatory increase in the other.
The hepatic artery has α1-adrenergic vasoconstriction receptors as well as β2-adrenergic, dopaminergic (D1), and cholinergic vasodilator receptors. The portal vein has only α1-adrenergic and dopaminergic (D1) receptors. Sympathetic activation results in vasoconstriction of the hepatic artery and mesenteric vessels, decreasing hepatic blood flow. β-Adrenergic stimulation vasodilates the hepatic artery; β-blockers reduce blood flow, and, therefore, decrease portal pressure.
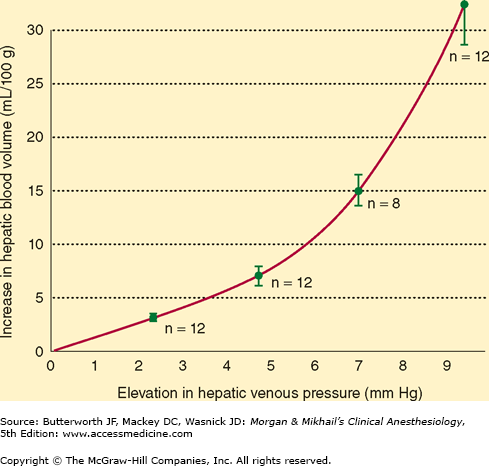
Portal vein pressure is normally only about 7-10 mm Hg, but the low resistance of the hepatic sinusoids allows relatively large blood flows through the portal vein. Small changes in hepatic venous tone and hepatic venous pressure thus can result in large changes in hepatic blood volume, allowing the liver to act as a blood reservoir (Figure 32-3). A decrease in hepatic venous pressure, as occurs during hemorrhage, shifts blood from hepatic veins and sinusoids into the central venous circulation and augments circulating blood volume. Blood loss can be reduced during liver surgery by lowering the central venous pressure, thereby reducing hepatic venous pressure and hepatic blood volume. In patients with congestive heart failure, the increase in central venous pressure is transmitted to the hepatic veins and causes congestion of the liver that can adversely affect liver function.
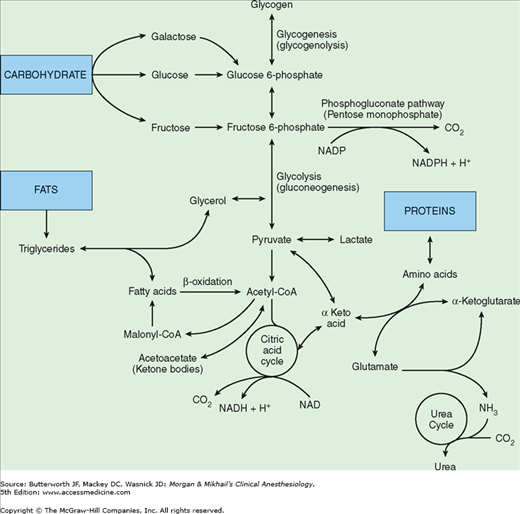
The abundance of enzymatic pathways in the liver allows it to play a key role in the metabolism of carbohydrates, fats, proteins, and other substances (see Figure 32-4 and Table 32-1). The final products of carbohydrate digestion are glucose, fructose, and galactose. With the exception of the large amount of fructose that is converted by the liver to lactate, hepatic conversion of fructose and galactose into glucose makes glucose metabolism the final common pathway for most carbohydrates.
Figure 32-4
Important metabolic pathways in hepatocytes. Although small amounts of adenosine triphosphate (ATP) are derived directly from some intermediary reactions, the overwhelming majority of ATP produced is the result of oxidative phosphorylation of the reduced forms of nicotinamide adenine dinucleotide (NADH) and nicotinamide adenine dinucleotide phosphate (NADPH).
| Creation and secretion of bile |
|
|
|
|
All cells utilize glucose to produce energy in the form of adenosine triphosphate (ATP), either aerobically via the citric acid cycle or anaerobically via glycolysis. The liver and adipose tissue can also utilize the phosphogluconate pathway, which provides energy and fatty acid synthesis. Most of the glucose absorbed following a meal is normally stored as glycogen, which only the liver and muscle are able to store in significant amounts. When glycogen storage capacity is exceeded, excess glucose is converted into fat. Insulin enhances glycogen synthesis, and epinephrine and glucagon enhance glycogenolysis. Because glucose consumption averages 150 g/day, and hepatic glycogen stores are normally only about 70 g/day, glycogen stores are depleted after 24 hr of fasting. After this period of fasting, gluconeogenesis, the de novo synthesis of glucose, is necessary to provide an uninterrupted supply of glucose for other organs.
The liver and kidney are unique in their capacity to form glucose from lactate, pyruvate, amino acids (mainly alanine), and glycerol (derived from fat metabolism). Hepatic gluconeogenesis is vital in the maintenance of a normal blood glucose concentration. Glucocorticoids, catecholamines, glucagon, and thyroid hormone greatly enhance gluconeogenesis, whereas insulin inhibits it.
When carbohydrate stores are saturated, the liver converts the excess ingested carbohydrates and proteins into fat. The fatty acids thus formed can be used immediately for fuel or stored in adipose tissue or the liver for later consumption. Nearly all cells utilize fatty acids derived from ingested fats or synthesized from intermediary metabolites of carbohydrates and protein as an energy source—only red blood cells and the renal medulla are limited to glucose utilization. Neurons normally utilize only glucose, but, after a few days of starvation, they can switch to ketone bodies, the breakdown products of fatty acids that have been synthesized by the liver as an energy source.
To oxidize fatty acids, they are converted into acetylcoenzyme A (acetyl-CoA), which is then oxidized via the citric acid cycle to produce ATP. The liver is capable of high rates of fatty acid oxidation and can form acetoacetic acid (one of the ketone bodies) from excess acetyl-CoA. The acetoacetate released by hepatocytes serves as an alternative energy source for other cell types by reconversion into acetyl-CoA. Insulin inhibits hepatic ketone body production. Acetyl-CoA is also used by the liver for the production of cholesterol and phospholipids, which is necessary in the synthesis of cellular membranes throughout the body.
The liver performs a critical role in protein metabolism. Without this function, death usually occurs within several days. The steps involved in protein metabolism include: (1) deamination of amino acids, (2) formation of urea (to eliminate the ammonia produced from deamination), (3) interconversions between nonessential amino acids, and (4) formation of plasma proteins. Deamination is necessary for the conversion of excess amino acids into carbohydrates and fats. The enzymatic processes, most commonly transamination, convert amino acids into their respective keto acids and produce ammonia as a byproduct.
Ammonia formed from deamination (as well as that produced by colonic bacteria and absorbed through the gut) is highly toxic to tissues. Through a series of enzymatic steps, the liver combines two molecules of ammonia with CO2 to form urea. The urea thus formed readily diffuses out of the liver and can then be excreted by the kidneys.
Nearly all plasma proteins, with the notable exception of immunoglobulins, are formed by the liver. These include albumin, α1-antitrypsin and other proteases/elastases, and the coagulation factors. Albumin is responsible for maintaining a normal plasma oncotic pressure and is the principal binding and transport protein for fatty acids and a large number of hormones and drugs. Consequently, changes in albumin concentration can affect the concentration of the pharmacologically active, unbound fraction of many drugs.
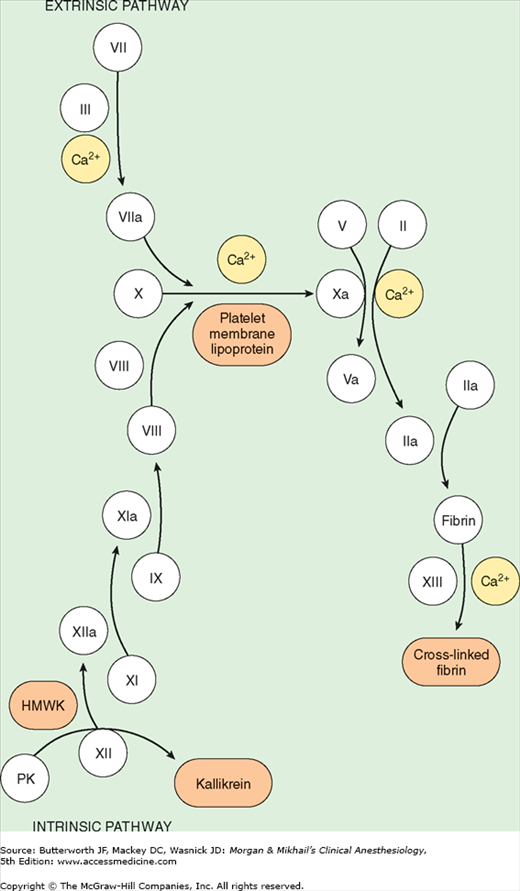
 All coagulation factors, with the exception of factor VIII and von Willebrand factor, are produced by the liver (see Table 32-2, Figure 32-5, and Chapter 51). Vascular endothelial cells synthesize factor VIII, levels of which are therefore usually maintained in chronic liver disease. Vitamin K is a necessary cofactor in the synthesis of prothrombin (factor II) and factors VII, IX, and X. The liver also produces plasma cholinesterase (pseudocholinesterase), an enzyme that hydrolyzes esters, including some local anesthetics and some muscle relaxants. Other important proteins formed by the liver include protease inhibitors (antithrombin III, α2-antiplasmin, and α1-antitrypsin), transport proteins (transferrin, haptoglobin, and ceruloplasmin), complement, α1-acid glycoprotein, C-reactive protein, and serum amyloid A.
All coagulation factors, with the exception of factor VIII and von Willebrand factor, are produced by the liver (see Table 32-2, Figure 32-5, and Chapter 51). Vascular endothelial cells synthesize factor VIII, levels of which are therefore usually maintained in chronic liver disease. Vitamin K is a necessary cofactor in the synthesis of prothrombin (factor II) and factors VII, IX, and X. The liver also produces plasma cholinesterase (pseudocholinesterase), an enzyme that hydrolyzes esters, including some local anesthetics and some muscle relaxants. Other important proteins formed by the liver include protease inhibitors (antithrombin III, α2-antiplasmin, and α1-antitrypsin), transport proteins (transferrin, haptoglobin, and ceruloplasmin), complement, α1-acid glycoprotein, C-reactive protein, and serum amyloid A.
| Factor | Approximate Half-Life (h) | |
|---|---|---|
| I | Fibrinogen | 100 |
| II | Prothrombin | 80 |
| III | Tissue thromboplastin | — |
| IV | Calcium | — |
| V | Proaccelerin | 18 |
| VII | Proconvertin | 6 |
| VIII | Antihemophilic factor | 10 |
| IX | Christmas factor | 24 |
| X | Stuart factor | 50 |
| XI | Plasma thromboplastin antecedents | 25 |
| XII | Hageman factor | 60 |
| XIII | Fibrin-stabilizing factor | 90 |
Many exogenous substances, including most drugs, undergo hepatic biotransformation, and the end-products of these reactions are usually either inactivated or converted to more water-soluble substances that can be readily excreted in bile or urine. Hepatic biotransformations are often categorized as one of two types of reactions. Phase I reactions modify reactive chemical groups through mixed-function oxidases or the cytochrome P-450 enzyme systems, resulting in oxidation, reduction, deamination, sulfoxidation, dealkylation, or methylation. Barbiturates and benzodiazepines are inactivated by phase I reactions. Phase II reactions, which may or may not follow a phase I reaction, involve conjugation of the substance with glucuronide, sulfate, taurine, or glycine. The conjugated compound can then be readily eliminated in urine or bile.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree