Key Concepts
 Cardiovascular complications account for 25% to 50% of deaths following noncardiac surgery. Perioperative myocardial infarction (MI), pulmonary edema, congestive heart failure, arrhythmias, and thromboembolism are most commonly seen in patients with preexisting cardiovascular disease.
Cardiovascular complications account for 25% to 50% of deaths following noncardiac surgery. Perioperative myocardial infarction (MI), pulmonary edema, congestive heart failure, arrhythmias, and thromboembolism are most commonly seen in patients with preexisting cardiovascular disease.
 Regardless of the level of preoperative blood pressure control, many patients with hypertension display an accentuated hypotensive response to induction of anesthesia, followed by an exaggerated hypertensive response to intubation. Hypertensive patients may display an exaggerated response to both endogenous catecholamines (from intubation or surgical stimulation) and exogenously administered sympathetic agonists.
Regardless of the level of preoperative blood pressure control, many patients with hypertension display an accentuated hypotensive response to induction of anesthesia, followed by an exaggerated hypertensive response to intubation. Hypertensive patients may display an exaggerated response to both endogenous catecholamines (from intubation or surgical stimulation) and exogenously administered sympathetic agonists.
 Patients with extensive (three-vessel or left main) coronary artery disease, a history of MI, or ventricular dysfunction are at greatest risk of cardiac complications.
Patients with extensive (three-vessel or left main) coronary artery disease, a history of MI, or ventricular dysfunction are at greatest risk of cardiac complications.
 Holter monitoring, exercise electrocardiography, myocardial perfusion scans, and echocardiography are important in determining perioperative risk and the need for coronary angiography; however, these tests are indicated only if their outcome would alter patient care.
Holter monitoring, exercise electrocardiography, myocardial perfusion scans, and echocardiography are important in determining perioperative risk and the need for coronary angiography; however, these tests are indicated only if their outcome would alter patient care.
 Sudden withdrawal of antianginal medication perioperatively—particularly β-blockers—can precipitate a sudden, rebound increase in ischemic episodes.
Sudden withdrawal of antianginal medication perioperatively—particularly β-blockers—can precipitate a sudden, rebound increase in ischemic episodes.
 The overwhelming priority in managing patients with ischemic heart disease is maintaining a favorable myocardial supply-demand relationship. Autonomic-mediated increases in heart rate and blood pressure should be controlled by deep anesthesia or adrenergic blockade, and excessive reductions in coronary perfusion pressure or arterial oxygen content are to be avoided.
The overwhelming priority in managing patients with ischemic heart disease is maintaining a favorable myocardial supply-demand relationship. Autonomic-mediated increases in heart rate and blood pressure should be controlled by deep anesthesia or adrenergic blockade, and excessive reductions in coronary perfusion pressure or arterial oxygen content are to be avoided.
 Intraoperative detection of ischemia depends on recognition of electrocardiographic changes, hemodynamic manifestations, or regional wall motion abnormalities on transesophageal echocardiography. New ST-segment elevations are rare during noncardiac surgery and are indicative of severe ischemia, vasospasm, or infarction.
Intraoperative detection of ischemia depends on recognition of electrocardiographic changes, hemodynamic manifestations, or regional wall motion abnormalities on transesophageal echocardiography. New ST-segment elevations are rare during noncardiac surgery and are indicative of severe ischemia, vasospasm, or infarction.
 The principal hemodynamic goals in managing mitral stenosis are to maintain a sinus rhythm (if present preoperatively) and to avoid tachycardia, large increases in cardiac output, and both hypovolemia and fluid overload by judicious administration of intravenous fluids.
The principal hemodynamic goals in managing mitral stenosis are to maintain a sinus rhythm (if present preoperatively) and to avoid tachycardia, large increases in cardiac output, and both hypovolemia and fluid overload by judicious administration of intravenous fluids.
 Anesthetic management of mitral regurgitation should be tailored to the severity of regurgitation and to the underlying left ventricular function. Factors that exacerbate the regurgitation, such as slow heart rates and acute increases in afterload, should be avoided. Excessive volume expansion can also worsen the regurgitation by dilating the left ventricle.
Anesthetic management of mitral regurgitation should be tailored to the severity of regurgitation and to the underlying left ventricular function. Factors that exacerbate the regurgitation, such as slow heart rates and acute increases in afterload, should be avoided. Excessive volume expansion can also worsen the regurgitation by dilating the left ventricle.
 Maintenance of normal sinus rhythm, heart rate, vascular resistance and intravascular volume is critical in patients with aortic stenosis. Loss of a normally timed atrial systole often leads to rapid deterioration, particularly when associated with tachycardia. Spinal and epidural anesthesia are relatively contraindicated in patients with severe aortic stenosis.
Maintenance of normal sinus rhythm, heart rate, vascular resistance and intravascular volume is critical in patients with aortic stenosis. Loss of a normally timed atrial systole often leads to rapid deterioration, particularly when associated with tachycardia. Spinal and epidural anesthesia are relatively contraindicated in patients with severe aortic stenosis.
 Bradycardia and increase in systemic vascular resistance (SVR) increase the regurgitant volume in patients with aortic regurgitation, whereas tachycardia can contribute to myocardial ischemia. Excessive myocardial depression should also be avoided. The compensatory increase in cardiac preload should be maintained, but excessive fluid replacement can readily result in pulmonary edema.
Bradycardia and increase in systemic vascular resistance (SVR) increase the regurgitant volume in patients with aortic regurgitation, whereas tachycardia can contribute to myocardial ischemia. Excessive myocardial depression should also be avoided. The compensatory increase in cardiac preload should be maintained, but excessive fluid replacement can readily result in pulmonary edema.
 In patients with congenital heart disease, an increase in SVR relative to pulmonary vascular resistance (PVR) favors left-to-right shunting, whereas an increase in PVR relative to SVR favors right-to-left shunting.
In patients with congenital heart disease, an increase in SVR relative to pulmonary vascular resistance (PVR) favors left-to-right shunting, whereas an increase in PVR relative to SVR favors right-to-left shunting.
 The presence of shunt flow between the right and left hearts, regardless of the direction of blood flow, mandates the meticulous exclusion of air bubbles or particulate material from intravenous fluids to prevent paradoxical embolism into the cerebral or coronary circulations.
The presence of shunt flow between the right and left hearts, regardless of the direction of blood flow, mandates the meticulous exclusion of air bubbles or particulate material from intravenous fluids to prevent paradoxical embolism into the cerebral or coronary circulations.
 The goals of anesthetic management in patients with tetralogy of Fallot should be to maintain intravascular volume and SVR. Increases in PVR, such as might occur from acidosis or excessive airway pressures, should be avoided. The right-to-left shunting tends to slow the uptake of inhalation anesthetics; in contrast, it may accelerate the onset of intravenous agents.
The goals of anesthetic management in patients with tetralogy of Fallot should be to maintain intravascular volume and SVR. Increases in PVR, such as might occur from acidosis or excessive airway pressures, should be avoided. The right-to-left shunting tends to slow the uptake of inhalation anesthetics; in contrast, it may accelerate the onset of intravenous agents.
 The transplanted heart is totally denervated, so direct autonomic influences are absent. Moreover, the absence of reflex increases in heart rate can make patients particularly sensitive to rapid vasodilatation. Indirect vasopressors, such as ephedrine, are less effective than direct-acting agents because of the absence of catecholamine stores in myocardial neurons.
The transplanted heart is totally denervated, so direct autonomic influences are absent. Moreover, the absence of reflex increases in heart rate can make patients particularly sensitive to rapid vasodilatation. Indirect vasopressors, such as ephedrine, are less effective than direct-acting agents because of the absence of catecholamine stores in myocardial neurons.
Anesthesia for Patients with Cardiovascular Disease: Introduction
Cardiovascular diseases—particularly hypertensive, ischemic, congenital, and valvular heart disease—are among the medical illnesses most frequently encountered in anesthetic practice and are a major cause of perioperative morbidity and mortality. Management of patients with these diseases continues to challenge the ingenuity and resources of the anesthesiologist. The adrenergic response to surgical stimulation and the circulatory effects of anesthetic agents, endotracheal intubation, positive-pressure ventilation, blood loss, fluid shifts, and alterations in body temperature impose additional burdens on an often already compromised cardiovascular system. Most anesthetic agents cause cardiac depression, vasodilatation, or both. Even anesthetics that have no direct circulatory effects may cause apparent circulatory depression in severely compromised patients who are dependent on the enhanced sympathetic activity characteristic of heart failure or acute blood loss. Decreased sympathetic activity as a consequence of the anesthetized state can lead to acute circulatory collapse.
Good anesthetic management of patients with cardiovascular disease requires a thorough knowledge of normal cardiac physiology, the circulatory effects of the various anesthetic agents, and the pathophysiology and treatment of these diseases. The same principles used in treating cardiovascular diseases in patients not undergoing surgery should be used perioperatively. In most instances, the choice of anesthetic agent is not terribly important; on the other hand, knowing how the agent is used, understanding the underlying pathophysiology, and understanding how the two interact are critical.
Patients with severe cardiovascular illnesses commonly undergo both cardiac and noncardiac surgery. The American College of Cardiology (ACC), in collaboration with the American Heart Association (AHA), have issued numerous guidelines related to the management of patients with heart disease, and many of their recommendations are relevant to patients undergoing anesthesia and invasive procedures. Because guidelines change as new evidence becomes available, anesthesiologists are advised to review the AHA website for current evidence-based indications for the management of heart disease.
Perioperative Cardiovascular Evaluation and Preparation for Noncardiac Surgery
The prevalence of cardiovascular disease increases progressively with advancing age. Moreover, the number of patients over 65 years of age is expected to increase by 25% to 35% over the next two decades.  Cardiovascular complications account for 25% to 50% of deaths following noncardiac surgery. Perioperative myocardial infarction (MI), pulmonary edema, systolic and diastolic heart failure, arrhythmias, and thromboembolism are the most common diagnoses in patients with preexisting cardiovascular disease. The incidence of postoperative cardiogenic pulmonary edema is approximately 2% in all patients over 40 years of age, but it is 6% in patients with a history of heart failure and 16% in patients with poorly compensated heart failure. The relatively high prevalence of cardiovascular disorders in surgical patients has given rise to attempts to define cardiac risk or the likelihood of intraoperative or postoperative fatal or life-threatening cardiac complications.
Cardiovascular complications account for 25% to 50% of deaths following noncardiac surgery. Perioperative myocardial infarction (MI), pulmonary edema, systolic and diastolic heart failure, arrhythmias, and thromboembolism are the most common diagnoses in patients with preexisting cardiovascular disease. The incidence of postoperative cardiogenic pulmonary edema is approximately 2% in all patients over 40 years of age, but it is 6% in patients with a history of heart failure and 16% in patients with poorly compensated heart failure. The relatively high prevalence of cardiovascular disorders in surgical patients has given rise to attempts to define cardiac risk or the likelihood of intraoperative or postoperative fatal or life-threatening cardiac complications.
In 2007, the ACC/AHA Task Force Report produced revised guidelines for perioperative evaluation. The revised guidelines stated that the patient’s medical history is critical in determining the requirements for preoperative cardiac evaluation and that certain conditions (eg, unstable coronary syndromes and decompensated heart failure) warrant cardiology intervention prior to all but emergency procedures (Table 21-1). The history should also review any past procedures, such as cardioverter defibrillator implants, coronary stents, and other interventions. Additionally, the patient’s ability to perform the tasks of daily living should be assessed as a guide to determine functional capacity. A patient with a history of cardiac disease and advanced age, but good exercise tolerance, will likely have a lower perioperative risk than a similar individual with dyspnea after minimal physical activity (Table 21-2).
| Condition | Examples |
|---|---|
| Unstable coronary syndromes |
|
| Decompensated HF (NYHA functional class IV; worsening or new-onset HF) | |
| Significant arrhythmias |
|
| Severe valvular disease |
|
The patient’s history should also seek signs of other disease processes that frequently accompany heart disease. Cardiac patients often present with obstructive pulmonary disease, reduced renal function, and diabetes mellitus.
A physical examination should be performed on all patients, and the heart and lungs should be auscultated. The physical examination is especially useful in patients with certain conditions. For example, if a murmur suggestive of aortic stenosis is detected, additional ultrasound evaluation will likely be warranted, as aortic stenosis substantially increases the risks in patients undergoing noncardiac surgery.
- Ischemic heart disease (history of MI, evidence on electrocardiogram [ECG], chest pain)
- Congestive heart failure (dyspnea, pulmonary edema)
- Cerebral vascular disease (stroke)
- High-risk surgery (vascular, thoracic, abdominal, orthopedic)
- Diabetes mellitus
- Preoperative creatinine >2 mg/dL
Recent ACC/AHA guidelines identify conditions that are a major cardiac risk and warrant intensive management prior to all but emergent surgery. These conditions include: unstable coronary syndromes (recent MI, unstable angina), decompensated heart failure, significant arrhythmias, and severe valvular heart disease. The ACC/AHA guidelines identify an MI within 7 days, or one within 1 month with myocardium at risk for ischemia, as “active” cardiac conditions. On the other hand, evidence of past MI with no myocardium thought at ischemic risk is considered a low risk for perioperative infarction after noncardiac surgery.
The ACC/AHA guidelines suggest a stepwise approach to preoperative cardiac evaluation. Their recommendations are classified as follows:
- Class I: Benefits >> risk
- Class IIa: Benefits >> risk, but scientific evidence incomplete
- Class IIb: Benefits ≥ risk, and scientific evidence incomplete
- Class III: Risks >>benefits
- Patients who have a need for emergency noncardiac surgery should proceed to the operating room with perioperative surveillance and postoperative risk factor management
- Patients with active cardiac conditions should be evaluated by a cardiologist and treated according to ACC/AHA guidelines
- Patients undergoing low-risk procedures should proceed to surgery
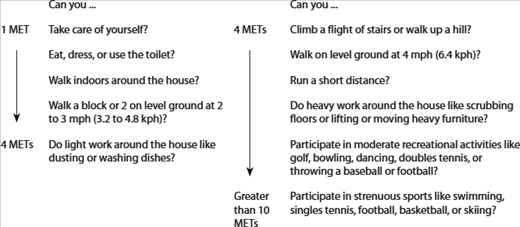
- Patients with poor exercise tolerance (<4 metabolic equivalents [METs]) and no known risk factors should proceed to surgery
- Patients with a functional capacity >4 METs and without symptoms should proceed to surgery
- Patients with a functional capacity <4 METs or those with an unknown functional capacity with three or more clinical risk factors scheduled for vascular surgery should be tested, if management is likely to change based on the results
- Patients with a functional capacity <4 METs or those with an unknown functional capacity with three or more clinical risk factors scheduled for intermediate-risk surgery should proceed to surgery with heart rate control
- Patients with a functional capacity <4 METs or those with an unknown functional capacity with one or two clinical risk factors who are scheduled for vascular or intermediate-risk surgery should proceed to surgery with heart rate control
The ACC/AHA guidelines also note, as class IIb recommendations, that noninvasive testing might be considered if patient management changes in patients with poor or unknown functional capacity or in patients undergoing intermediate-risk surgery with three clinical risk factors. Likewise, such testing might be indicated in patients with one or two clinical risk factors scheduled for vascular or intermediate-risk surgery. Table 21-3 classifies surgical procedures according to risk.
| Risk Stratification | Procedure Examples |
|---|---|
| Vascular (reported cardiac risk often more than 5%) |
|
| Intermediate (reported cardiac risk generally 1% to 5%) |
|
| Low2 (reported cardiac risk generally less than 1%) |
|
The ACC/AHA guidelines also provide specific recommendations regarding various conditions likely to be encountered perioperatively.
The ACC/AHA guidelines note that only the subset of patients with coronary artery disease (CAD) who would benefit from revascularization, irrespective of their need for a nonemergent surgical procedure, would likely benefit from preoperative coronary interventions. Consequently, the indications for the testing of such patients as candidates for a coronary intervention are unrelated to their presenting for surgery and depend only on whether such evaluation would be indicated as part of general medical management.
Patients with hypertension frequently present for elective surgical procedures. Some will have been effectively managed, but unfortunately, many others will not have been. Hypertension is a leading cause of death and disability in most Western societies and the most prevalent preoperative medical abnormality in surgical patients, with an overall prevalence of 20% to 25%. Long-standing uncontrolled hypertension accelerates atherosclerosis and hypertensive organ damage. Hypertension is a major risk factor for cardiac, cerebral, renal, and vascular disease. Complications of hypertension include MI, congestive heart failure, stroke, renal failure, peripheral occlusive disease, and aortic dissection. The presence of left ventricular hypertrophy (LVH) in hypertensive patients may be an important predictor of cardiac mortality. However, systolic blood pressures below 180 mm Hg, and diastolic pressures below 110 mm Hg, have not been associated with increased perioperative risks. When patients present with systolic blood pressures greater than 180 mm Hg and diastolic pressures greater than 110 mm Hg, anesthesiologists face the dilemma of delaying surgery to allow optimization of oral antihypertensive therapy, but adding the risk of a surgical delay versus proceeding with surgery and achieving blood pressure control with rapidly acting intravenous agents. Intravenous β-blockers can be useful to treat preoperative hypertension. Of note, patients with preoperative hypertension are more likely than others to develop intraoperative hypotension. This is particularly frequent in patients treated with angiotensin receptor blockers and/or angiotensin-converting enzyme (ACE) inhibitors.
Blood pressure measurements are affected by many variables, including posture, time of day or night, emotional state, recent activity, and drug intake, as well as the equipment and technique used. A diagnosis of hypertension cannot be made by one preoperative reading, but requires confirmation by a history of consistently elevated measurements. Although preoperative anxiety or pain may produce some degree of hypertension in normal patients, patients with a history of hypertension generally exhibit greater preoperative elevations in blood pressure.
Epidemiological studies demonstrate a direct and continuous correlation between both diastolic and systolic blood pressures and mortality rates. The definition of systemic hypertension is arbitrary: a consistently elevated diastolic blood pressure greater than 90 mm Hg or a systolic pressure greater than 140 mm Hg. A common classification scheme is listed in Table 21-4. Borderline hypertension is said to exist when the diastolic pressure is 85-89 mm Hg or the systolic pressure is 130-139 mm Hg. Whether patients with borderline hypertension are at some increased risk for cardiovascular complications remains unclear. Accelerated, or severe hypertension (stage 3), is defined as a recent, sustained, and progressive increase in blood pressure, usually with diastolic blood pressures in excess of 110-119 mm Hg. Renal dysfunction is often present in such patients. Malignant hypertension is a true medical emergency characterized by severe hypertension (>210/120 mm Hg) often associated with papilledema and encephalopathy.
| Category of Blood Pressure | Systolic Pressure (mm Hg) | Diastolic Pressure (mm Hg) |
|---|---|---|
| Normal | <130 | <85 |
| High normal | 130-139 | 85-89 |
| Hypertension | ||
| Stage 1/mild | 140-159 | 90-99 |
| Stage 2/moderate | 160-179 | 100-109 |
| Stage 3/severe | 180-209 | 110-119 |
| Stage 4/very severe | >210 | >120 |
Hypertension can be either idiopathic (essential), or, less commonly, secondary to other medical conditions such as renal disease, renal artery stenosis, primary hyperaldosteronism, Cushing’s disease, acromegaly, pheochromocytoma, pregnancy, or estrogen therapy. Essential hypertension accounts for 80% to 95% of cases and may be associated with an abnormal baseline elevation of cardiac output, systemic vascular resistance (SVR), or both. An evolving pattern is commonly seen over the course of the disease, where cardiac output returns to (or remains) normal, but SVR becomes abnormally high. The chronic increase in cardiac afterload results in concentric LVH and altered diastolic function. Hypertension also alters cerebral autoregulation, such that normal cerebral blood flow is maintained in the face of high blood pressures; autoregulation limits may be in the range of mean blood pressures of 110-180 mm Hg.
The mechanisms responsible for the changes observed in hypertensive patients seem to involve vascular hypertrophy, hyperinsulinemia, abnormal increases in intracellular calcium, and increased intracellular sodium concentrations in vascular smooth muscle and renal tubular cells. The increased intracellular calcium presumably results in increased arteriolar tone, whereas the increased sodium concentration impairs renal excretion of sodium. Sympathetic nervous system overactivity and enhanced responses to sympathetic agonists are present in some patients. Hypertensive patients sometimes display an exaggerated response to vasopressors and vasodilators. Overactivity of the renin-angiotensin-aldosterone system seems to play an important role in patients with accelerated hypertension.
Effective drug therapy reduces the progression of hypertension and the incidence of stroke, congestive heart failure, CAD, and renal damage. Effective treatment can also delay and sometimes reverse concomitant pathophysiological changes, such as LVH and altered cerebral autoregulation.
Some patients with mild hypertension require only single-drug therapy, which may consist of a thiazide diuretic, ACE inhibitor, angiotensin-receptor blocker (ARB), β-adrenergic blocker, or calcium channel blocker, although guidelines and outcome studies favor the first three options. Concomitant illnesses should guide drug selection. All patients with a prior MI should receive a β-adrenergic blocker and an ACE inhibitor (or ARB) to improve outcomes, irrespective of the presence of hypertension. In many patients, the “guideline specified” agents will also be more than sufficient to control hypertension.
Patients with moderate to severe hypertension often require two or three drugs for control. The combination of a diuretic with a β-adrenergic blocker and an ACE inhibitor is often effective when single-drug therapy is not. As previously noted, ACE inhibitors (or ARBs) prolong survival in patients with congestive heart failure, left ventricular dysfunction, or a prior MI. Familiarity with the names, mechanisms of action, and side effects of commonly used antihypertensive agents is important for anesthesiologists (Table 21-5).
| Category | Class | Subclass | Agent |
|---|---|---|---|
| Diuretics | Thiazide |
| |
| Potassium sparing |
| ||
| Loop |
| ||
| Sympatholytics | Adrenergic-receptor blockers | B |
|
| A |
| ||
| α and β |
| ||
| Central α2-agonists |
| ||
| Vasodilators | Calcium channel blockers | Benzothiazepine | |
| Diltiazem1 (Tiazac) | |||
| Phenylalkylamines | |||
| Verapamil1 (Calan SR) | |||
| Dihydropyridines | |||
| |||
| ACE inhibitors2 |
| ||
| Angiotensin-receptor antagonists |
| ||
| Direct vasodilators |
|
A recurring question in anesthetic practice is the degree of preoperative hypertension that is acceptable for patients scheduled for elective surgery. Except for optimally controlled patients, most hypertensive patients present to the operating room with some degree of hypertension. Although data suggest that even moderate preoperative hypertension (diastolic pressure <90-110 mm Hg) is not clearly statistically associated with postoperative complications, other data indicate that the untreated or poorly controlled hypertensive patient is more apt to experience intraoperative episodes of myocardial ischemia, arrhythmias, or both hypertension and hypotension. Intraoperative adjustments in anesthetic depth and use of vasoactive drugs should reduce the incidence of postoperative complications referable to poor preoperative control of hypertension.
Although patients should ideally undergo elective surgery only when rendered normotensive, this is not always feasible or necessarily desirable because of altered cerebral autoregulation. Excessive reductions in blood pressure can compromise cerebral perfusion. Moreover, the decision to delay or to proceed with surgery should be individualized, based on the severity of the preoperative blood pressure elevation; the likelihood of coexisting myocardial ischemia, ventricular dysfunction, or cerebrovascular or renal complications; and the surgical procedure (whether major surgically induced changes in cardiac preload or afterload are anticipated). With rare exceptions, antihypertensive drug therapy should be continued up to the time of surgery. Some clinicians withhold ACE inhibitors and ARBs on the morning of surgery because of their association with an increased incidence of intraoperative hypotension; however, withholding these agents increases the risk of marked perioperative hypertension and the need for parenteral antihypertensive agents. It also requires the surgical team to remember to restart the medication after surgery. The decision to delay elective surgical procedures in patients with sustained preoperative diastolic blood pressures higher than 110 mm Hg should be made when the perceived benefits of delayed surgery exceed the risks. Unfortunately, there are few appropriate studies to guide the decision-making.
The preoperative history should inquire into the severity and duration of the hypertension, the drug therapy currently prescribed, and the presence or absence of hypertensive complications. Symptoms of myocardial ischemia, ventricular failure, impaired cerebral perfusion, or peripheral vascular disease should be elicited, as well as the patient’s record of compliance with the drug regimen. The patient should be questioned regarding chest pain, exercise tolerance, shortness of breath (particularly at night), dependent edema, postural lightheadedness, syncope, episodic visual disturbances or episodic neurologic symptoms, and claudication. Adverse effects of current antihypertensive drug therapy (Table 21-6) should also be identified.
| Class | Adverse Effects |
|---|---|
| Diuretics | |
| Thiazide | Hypokalemia, hyponatremia, hyperglycemia, hyperuricemia, hypomagnesemia, hyperlipidemia, hypercalcemia |
| Loop | Hypokalemia, hyperglycemia, hypocalcemia, hypomagnesemia, metabolic alkalosis |
| Potassium sparing | Hyperkalemia |
| Sympatholytics | |
| β-Adrenergic blockers | Bradycardia, conduction blockade, myocardial depression, enhanced bronchial tone, sedation, fatigue, depression |
| α-Adrenergic blockers | Postural hypertension, tachycardia, fluid retention |
| Central α2-agonists | Postural hypotension, sedation, dry mouth, depression, decreased anesthetic requirements, bradycardia, rebound hypertension, positive Coombs test and hemolytic anemia (methyldopa), hepatitis (methyldopa) |
| Ganglionic blockers | Postural hypotension, diarrhea, fluid retention, depression (reserpine) |
| Vasodilators | |
| Calcium channels blockers | Cardiac depression, bradycardia, conduction blockade (verapamil, diltiazem), peripheral edema (nifedipine), tachycardia (nifedipine), enhanced neuromuscular nondepolarizing blockade |
| ACE inhibitors1 | Cardiac depression, bradycardia, conduction blockade (verapamil, diltiazem), peripheral edema (nifedipine), tachycardia (nifedipine), enhanced neuromuscular nondepolarizing blockade |
| Angiotensin-receptor antagonists | Hypotension, renal failure in bilateral renal artery stenosis, hyperkalemia |
| Direct vasodilators | Reflex tachycardia, fluid retention, headache, systemic lupus erythematosus-like syndrome (hydralazine), pleural or pericardial effusion (minoxidil) |
Ophthalmoscopy is useful in hypertensive patients. Visible changes in the retinal vasculature usually parallel the severity and progression of arteriosclerosis and hypertensive damage in other organs. An S4 cardiac gallop is common in patients with LVH. Other physical findings, such as pulmonary rales and an S3 cardiac gallop, are late findings and indicate congestive heart failure. Blood pressure can be measured in both the supine and standing positions. Orthostatic changes can be due to volume depletion, excessive vasodilatation, or sympatholytic drug therapy; preoperative fluid administration can prevent severe hypotension after induction of anesthesia in these patients. Although asymptomatic carotid bruits are usually hemodynamically insignificant, they may be reflective of atherosclerotic vascular disease that may affect the coronary circulation. When a bruit is detected, further workup should be guided by the urgency of the scheduled surgery and the likelihood that further investigations, if diagnostic, would result in a change in therapy. Doppler studies of the carotid arteries can be used to define the extent of carotid disease.
The ECG is often normal, but in patients with a long history of hypertension, it may show evidence of ischemia, conduction abnormalities, an old infarction, or LVH or strain. A normal ECG does not exclude CAD or LVH. Similarly, a normal heart size on a chest radiograph does not exclude ventricular hypertrophy. Echocardiography is a sensitive test of LVH and can be used to evaluate ventricular systolic and diastolic functions in patients with symptoms of heart failure. Chest radiographs are rarely useful in an asymptomatic patient, but may show a boot-shaped heart (suggestive of LVH), frank cardiomegaly, or pulmonary vascular congestion.
Renal function is best evaluated by measurement of serum creatinine and blood urea nitrogen levels. Serum electrolyte levels (K) should be determined in patients taking diuretics or digoxin or those with renal impairment. Mild to moderate hypokalemia (3-3.5 mEq/L) is often seen in patients taking diuretics, but does not have adverse outcome effects. Potassium replacement should be undertaken only in patients who are symptomatic or who are also taking digoxin. Hypomagnesemia is often present and may be a cause of perioperative arrhythmias. Hyperkalemia may be encountered in patients who are taking potassium-sparing diuretics or ACE inhibitors, particularly those with impaired renal function.
Premedication reduces preoperative anxiety and is desirable in hypertensive patients. Mild to moderate preoperative hypertension often resolves following administration of an agent such as midazolam.
The overall anesthetic plan for a hypertensive patient is to maintain an appropriate stable blood pressure range. Patients with borderline hypertension may be treated as normotensive patients. Those with long-standing or poorly controlled hypertension, however, have altered autoregulation of cerebral blood flow; higher than normal mean blood pressures may be required to maintain adequate cerebral blood flow. Because most patients with long-standing hypertension are assumed to have some element of CAD and cardiac hypertrophy, excessive blood pressure elevations are undesirable. Hypertension, particularly in association with tachycardia, can precipitate or exacerbate myocardial ischemia, ventricular dysfunction, or both. Arterial blood pressure should generally be kept within 20% of preoperative levels. If marked hypertension (>180/120 mm Hg) is present preoperatively, arterial blood pressure should be maintained in the high-normal range (150-140/90-80 mm Hg).
Most hypertensive patients do not require special intraoperative monitors. Direct intraarterial pressure monitoring should be reserved for patients with wide swings in blood pressure and those undergoing major surgical procedures associated with rapid or marked changes in cardiac preload or afterload. Electrocardiographic monitoring should focus on detecting signs of ischemia. Urinary output should generally be monitored with an indwelling urinary catheter in patients with a preexisting renal impairment who are undergoing procedures expected to last more than 2 hr. When invasive hemodynamic monitoring is used, reduced ventricular compliance (see Chapter 20) is often apparent in patients with ventricular hypertrophy; these patients may require more intravenous fluid to produce a higher filling pressure to maintain adequate left ventricular end-diastolic volume and cardiac output. Volume administration in patients with decreased ventricular compliance can also result in elevated pulmonary arterial pressures and pulmonary congestion.
Induction of anesthesia and endotracheal intubation are often associated with hemodynamic instability in hypertensive patients.  Regardless of the level of preoperative blood pressure control, many patients with hypertension display an accentuated hypotensive response to induction of anesthesia, followed by an exaggerated hypertensive response to intubation. Many, if not most, antihypertensive agents and general anesthetics are vasodilators, cardiac depressants, or both. In addition, many hypertensive patients present for surgery in a volume-depleted state. Sympatholytic agents attenuate the normal protective circulatory reflexes, reducing sympathetic tone and enhancing vagal activity.
Regardless of the level of preoperative blood pressure control, many patients with hypertension display an accentuated hypotensive response to induction of anesthesia, followed by an exaggerated hypertensive response to intubation. Many, if not most, antihypertensive agents and general anesthetics are vasodilators, cardiac depressants, or both. In addition, many hypertensive patients present for surgery in a volume-depleted state. Sympatholytic agents attenuate the normal protective circulatory reflexes, reducing sympathetic tone and enhancing vagal activity.
Up to 25% of hypertensive patients may exhibit severe hypertension following endotracheal intubation. Prolonged laryngoscopy should be avoided. Moreover, intubation should generally be performed under deep anesthesia (provided hypotension can be avoided). One of several techniques may be used before intubation to attenuate the hypertensive response:
- Deepening anesthesia with a potent volatile agent
- Administering a bolus of an opioid (fentanyl, 2.5-5 mcg/kg; alfentanil, 15-25 mcg/kg; sufentanil, 0.5-1.0 mcg/kg; or remifentanil, 0.5-1 mcg/kg).
- Administering lidocaine, 1.5 mg/kg intravenously, intratracheally, or topically in the airway
- Achieving β-adrenergic blockade with esmolol, 0.3-1.5 mg/kg; metoprolol 1-5 mg; or labetalol, 5-20 mg.
The superiority of any one agent or technique over another has not been established. Propofol, barbiturates, benzodiazepines, and etomidate are equally safe for inducing general anesthesia in most hypertensive patients. Ketamine by itself can precipitate marked hypertension; however, it is almost never used as a single agent. When administered with a small dose of another agent, such as a benzodiazepine or propofol, ketamine’s sympathetic stimulating properties can be blunted or eliminated.
Anesthesia may be safely continued with volatile agents (alone or with nitrous oxide), a balanced technique (opioid + nitrous oxide + muscle relaxant), or a total intravenous technique. Regardless of the primary maintenance technique, addition of a volatile agent or intravenous vasodilator generally allows convenient intraoperative blood pressure control.
With the possible exception of large bolus doses of pancuronium, any muscle relaxant can be used. Pancuronium-induced vagal blockade and neural release of catecholamines could exacerbate hypertension in poorly controlled patients, but, if given slowly, in small increments, pancuronium is unlikely to cause medically important increases in heart rate or blood pressure. Moreover, pancuronium can be useful in offsetting excessive vagal tone induced by opioids or surgical manipulations. Hypotension following large (intubating) doses of atracurium may be accentuated in hypertensive patients.
Hypertensive patients may display an exaggerated response to both endogenous catecholamines (from intubation or surgical stimulation) and exogenously administered sympathetic agonists. If a vasopressor is necessary to treat excessive hypotension, a small dose of a direct-acting agent, such as phenylephrine (25-50 mcg), may be useful. Patients taking sympatholytics preoperatively may exhibit a decreased response to ephedrine. Vasopressin as a bolus or infusion can also be employed to restore vascular tone in the hypotensive patient.
Intraoperative hypertension not responding to an increase in anesthetic depth (particularly with a volatile agent) can be treated with a variety of parenteral agents (Table 21-7). Readily reversible causes—such as inadequate anesthetic depth, hypoxemia, or hypercapnia—should always be excluded before initiating antihypertensive therapy. Selection of a hypotensive agent depends on the severity, acuteness, and cause of hypertension; the baseline ventricular function; the heart rate; the presence of bronchospastic pulmonary disease; and the anesthetist’s familiarity with each of the drug options. β-Adrenergic blockade alone or as a supplement is a good choice for a patient with good ventricular function and an elevated heart rate, but is relatively contraindicated in a patient with bronchospastic disease. Metoprolol, esmolol, or labetolol are readily used intraoperatively. Nicardipine or clevidipine may be preferable to β-blockers for patients with bronchospastic disease. Nitroprusside remains the most rapid and effective agent for the intraoperative treatment of moderate to severe hypertension. Nitroglycerin may be less effective, but is also useful in treating or preventing myocardial ischemia. Fenoldopam, a dopamine agonist, is also a useful hypotensive agent; furthermore, it increases renal blood flow. Hydralazine provides sustained blood pressure control, but also has a delayed onset and can cause reflex tachycardia. The latter is not seen with labetalol because of a combined α- and β-adrenergic blockade.
| Agent | Dosage Range | Onset | Duration |
|---|---|---|---|
| Nitroprusside | 0.5-10 mcg/kg/min | 30-60 | 1-5 min |
| Nitroglycerin | 0.5-10 mcg/kg/min | 1 min | 3-5 min |
| Esmolol |
| 1 min | 12-20 min |
| Labetalol | 5-20 mg | 1-2 min | 4-8 hr |
| Metoprolol | 2.5-5 mg | 1-5 min | 5-8 hr |
| Hydralazine | 5-20 mg | 5-20 min | 4-8 hr |
| Clevidipine | 1-32 mg/hr | 1-3 min | 5-15 min |
| Nicardipine |
| 1-5 min | 3-4 hr |
| Enalaprilat | 0.625-1.25 mg | 6-15 min | 4-6 hr |
| Fenoldopam | 0.1-1.6 mg/kg/min | 5 min | 5 min |
Postoperative hypertension is common and should be anticipated in patients who have poorly controlled hypertension. Close blood pressure monitoring should be continued in both the recovery room and the early postoperative period. In addition to myocardial ischemia and congestive heart failure, marked sustained elevations in blood pressure can contribute to the formation of wound hematomas and the disruption of vascular suture lines.
Hypertension in the recovery period is often multifactorial and enhanced by respiratory abnormalities, anxiety and pain, volume overload, or bladder distention. Contributing causes should be corrected and parenteral antihypertensive agents given if necessary. Intravenous labetalol is particularly useful in controlling hypertension and tachycardia, whereas vasodilators are useful in controlling blood pressure in the setting of a slow heart rate. When the patient resumes oral intake, preoperative medications should be restarted.
Myocardial ischemia is characterized by a metabolic oxygen demand that exceeds the oxygen supply. Ischemia can therefore result from a marked increase in myocardial metabolic demand, a reduction in myocardial oxygen delivery, or a combination of both. Common causes include coronary arterial vasospasm or thrombosis; severe hypertension or tachycardia (particularly in the presence of ventricular hypertrophy); severe hypotension, hypoxemia, or anemia; and severe aortic stenosis or regurgitation.
By far, the most common cause of myocardial ischemia is atherosclerosis of the coronary arteries. CAD is responsible for about 25% of all deaths in Western societies and is a major cause of perioperative morbidity and mortality. The overall incidence of CAD in surgical patients is estimated to be between 5% and 10%. Major risk factors for CAD include hyperlipidemia, hypertension, diabetes, cigarette smoking, increasing age, male sex, and a positive family history. Other risk factors include obesity, a history of cerebrovascular or peripheral vascular disease, menopause, use of high-estrogen oral contraceptives (in women who smoke), and a sedentary lifestyle.
CAD may be clinically manifested by symptoms of myocardial necrosis (infarction), ischemia (usually angina), arrhythmias (including sudden death), or ventricular dysfunction (congestive heart failure). When symptoms of congestive heart failure predominate, the term “ischemic cardiomyopathy” is often used.
Unstable angina is defined as (1) an abrupt increase in severity, frequency (more than three episodes per day), or duration of anginal attacks (crescendo angina): (2) angina at rest; or (3) new onset of angina (within the past 2 months) with severe or frequent episodes (more than three per day). Unstable angina may occur following MI or be precipitated by noncardiac medical conditions (including severe anemia, fever, infections, thyrotoxicosis, hypoxemia, and emotional distress) in previously stable patients.
Unstable angina, particularly when it is associated with significant ST-segment changes at rest, usually reflects severe underlying coronary disease and frequently precedes MI. Plaque disruption with platelet aggregates or thrombi and vasospasm are frequent pathological correlates. Critical stenosis in one or more major coronary arteries is present in more than 80% of patients with these symptoms. Patients with unstable angina require evaluation and treatment, which may include admission to a coronary care unit and some form of coronary intervention.
Anginal chest pains are most often substernal, exertional, radiating to the neck or arm, and relieved by rest or nitroglycerin. Variations are common, including epigastric, back, or neck pain, or transient shortness of breath from ventricular dysfunction (anginal equivalent). Nonexertional ischemia and silent (asymptomatic) ischemia are recognized as fairly common occurrences. Patients with diabetes have an increased incidence of silent ischemia.
Symptoms are generally absent until the atherosclerotic lesions cause 50% to 75% occlusion of the coronary circulation. When a stenotic segment reaches 70% occlusion, maximum compensatory dilatation is usually present distally: blood flow is generally adequate at rest, but becomes inadequate with increased metabolic demand. An extensive collateral blood supply allows some patients to remain relatively asymptomatic in spite of severe disease. Coronary vasospasm is also a cause of transient transmural ischemia in some patients; 90% of vasospastic episodes occur at preexisting stenotic lesions in epicardial vessels and are often precipitated by a variety of factors, including emotional upset and hyperventilation (Prinzmetal’s angina). Coronary spasm is most often observed in patients who have angina with varying levels of activity or emotional stress (variable-threshold); it is least common with classic exertional (fixed-threshold) angina.
The overall prognosis of patients with CAD is related to both the number and severity of coronary obstructions, as well as to the extent of ventricular dysfunction.
The general approach in treating patients with ischemic heart disease is five-fold:
- Correction of risk factors, with the hope of slowing disease progression.
- Modification of the patient’s lifestyle to reduce stress and improve exercise tolerance.
- Correction of complicating medical conditions that can exacerbate ischemia (ie, hypertension, anemia, hypoxemia, hyperthyroidism, fever, infection, or adverse drug effects).
- Pharmacological manipulation of the myocardial oxygen supply-demand relationship.
- Correction of coronary lesions by percutaneous coronary intervention (angioplasty [with or without stenting] or atherectomy) or coronary artery bypass surgery.
The last three approaches are of direct relevance to anesthesiologists. The same principles should be applied in the care of these patients in both the operating room and the intensive care unit.
The most commonly used pharmacological agents are nitrates, β-blockers, and calcium channel blockers. These drugs also have potent circulatory effects, which are compared in Table 21-8. Any of these agents can be used for mild angina. Calcium channel blockers are the drugs of choice for patients with predominantly vasospastic angina. β-Blockers improve the long-term outcome of patients with CAD. Nitrates are good agents for both types of angina.
| Calcium Channel Blockers | |||||
|---|---|---|---|---|---|
| Cardiac Parameter | Nitrates | Verapamil |
| Diltiazem | β-Blockers |
| Preload | ↓↓ | — | — | — | —/↑ |
| Afterload | ↓ | ↓ | ↓↓ | ↓ | —/↓ |
| Contractility | — | ↓↓ | — | ↓ | ↓↓↓ |
| SA node automaticity | ↑/— | ↓↓ | ↑/— | ↓↓ | ↓↓↓ |
| AV conduction | — | ↓↓↓ | — | ↓↓ | ↓↓↓ |
| Vasodilatation | |||||
| Coronary | ↑ | ↑↑ | ↑↑↑ | ↑↑ | —/↓ |
| Systemic | ↑↑ | ↑ | ↑↑ | ↑ | —/↓ |
Nitrates relax all vascular smooth muscle, but have a much greater effect on venous than on arterial vessels. Decreasing venous and arteriolar tone and reducing the effective circulating blood volume (cardiac preload) reduce wall tension afterload. These effects tend to reduce myocardial oxygen demand. The prominent venodilatation makes nitrates excellent agents when congestive heart failure is also present.
Perhaps equally important, nitrates dilate the coronary arteries. Even minor degrees of dilatation at stenotic sites may be sufficient to increase blood flow, because flow is directly related to the fourth power of the radius. Nitrate-induced coronary vasodilatation preferentially increases subendocardial blood flow in ischemic areas. This favorable redistribution of coronary blood flow to ischemic areas may be dependent on the presence of collaterals in the coronary circulation.
Nitrates can be used for both the treatment of acute ischemia and prophylaxis against frequent anginal episodes. Unlike β-blockers and calcium channel blockers, nitrates do not have a negative inotropic effect—a desirable feature in the presence of ventricular dysfunction. Intravenous nitroglycerin can also be used for controlled hypotensive anesthesia.
The effects and uses of the most commonly used calcium channel blockers are shown in Table 21-9. Calcium channel blockers reduce myocardial oxygen demand by decreasing cardiac afterload and augment oxygen supply by increasing blood flow (coronary vasodilatation). Verapamil and diltiazem also reduce demand by slowing the heart rate.
| Clinical Use | ||||||||
|---|---|---|---|---|---|---|---|---|
| Agent | Route | Dosage1 | Half-life | Angina | Hypertension | Cerebral Vasospasm | Supraventricular Tachycardia | |
| Verapamil | PO | 40-240 mg | 5 hr | + | + | + | ||
| IV | 5-15 mg | 5 hr | + | + | ||||
| Nifedipine | PO | 30-180 mg | 2 hr | + | + | |||
| SL | 10 mg | 2 hr | + | + | ||||
| Diltiazem | PO | 30-60 mg | 4 hr | + | + | + | ||
| IV | 0.25-0.35 mg/kg | 4 hr | + | + | ||||
| Nicardipine | PO | 60-120 mg | 2-4 hr | + | + | |||
| IV | 0.25-0.5 mg/kg | 2-4 hr | + | + | ||||
| Nimodipine | PO | 240 mg | 2 hr | + | ||||
| Bepridil2 | PO | 200-400 mg | 24 hr | + | + | |||
| Isradipine | PO | 2.5-5.0 mg | 8 hr | + | ||||
| Felodipine | PO | 5-20 mg | 9 hr | + | ||||
| Amlodipine | PO | 2.5-10 mg | 30-50 hr | + | + | |||
Nifedipine’s potent effects on the systemic blood pressure may precipitate hypotension, reflex tachycardia, or both; its fast-onset preparations (eg, sublingual) have been associated with MI in some patients. Its tendency to decrease afterload generally offsets any negative inotropic effect. The slow-release form of nifedipine is associated with much less reflex tachycardia and is more suitable than other agents for patients with ventricular dysfunction. In contrast, verapamil and diltiazem have greater effects on cardiac contractility and atrioventricular (AV) conduction and therefore should be used cautiously, if at all, in patients with ventricular dysfunction, conduction abnormalities, or bradyarrhythmias. Diltiazem seems to be better tolerated than verapamil in patients with impaired ventricular function. Nicardipine, nimodipine, and clevidipine generally have the same effects as nifedipine; nimodipine is primarily used in preventing cerebral vasospasm following subarachnoid hemorrhage, whereas nicardipine is used as an intravenous arterial vasodilator. Clevidipine is an ultrashort-acting arterial vasodilator.
Calcium channel blockers can have significant interactions with anesthetic agents. All calcium channel blockers potentiate both depolarizing and nondepolarizing neuromuscular blocking agents and the circulatory effects of volatile agents. Both verapamil and diltiazem can potentiate depression of cardiac contractility and conduction in the AV node by volatile anesthetics. Nifedipine and similar agents can potentiate systemic vasodilatation by volatile and intravenous agents.
These drugs decrease myocardial oxygen demand by reducing heart rate and contractility, and, in some cases, afterload (via their antihypertensive effect). Optimal blockade results in a resting heart rate between 50 and 60 beats/min and prevents appreciable increases with exercise (<20 beats/min increase during exercise). Available agents differ in receptor selectivity, intrinsic sympathomimetic (partial agonist) activity, and membrane-stabilizing properties (Table 21-10). Membrane stabilization, often described as a quinidine-like effect, results in antiarrhythmic activity. Agents with intrinsic sympathomimetic properties are better tolerated by patients with mild to moderate ventricular dysfunction. Certain β-blockers (carvedilol and extended-duration metoprolol) improve survival in patients with chronic heart failure. This has not been shown to be a drug class effect. Blockade of β2-adrenergic receptors also can mask hypoglycemic symptoms in patients with diabetes, delay metabolic recovery from hypoglycemia, and impair the handling of large potassium loads. Cardioselective (β1-receptor-specific) agents, although generally better tolerated than nonselective agents in patients with reactive airways, must still be used cautiously in such patients. The selectivity of cardioselective agents tends to be dose dependent. Patients on long-standing β-blocker therapy should have these agents continued perioperatively. Acute β-blocker withdrawal in the perioperative period places patients at a markedly increased risk of cardiac morbidity and mortality.
| Agent | β1-Receptor Selectivity | Half-life | Sympathomimetic | α-Receptor Blockade | Membrane Stabilizing |
|---|---|---|---|---|---|
| Acebutolol | + | 2-4 hr | + | + | |
| Atenolol | ++ | 5-9 hr | |||
| Betaxlol | ++ | 14-22 hr | |||
| Esmolol | ++ | 9 min | |||
| Metoprolol | ++ | 3-4 hr | ± | ||
| Bisoprolol | + | 9-12 hr | |||
| Oxprenolol | 1-2 hr | + | + | ||
| Alprenolol | 2-3 hr | + | + | ||
| Pindolol | 3-4 hr | ++ | ± | ||
| Penbutolol | 5 hr | + | + | ||
| Carteolol | 6 hr | + | |||
| Labetalol | 4-8 hr | + | ± | ||
| Propranolol | 3-6 hr | ++ | |||
| Timolol | 3-5 hr | ||||
| Sotalol1 | 5-13 hr | ||||
| Nadolol | 10-24 hr | ||||
| Carvedilol | 6-8 hr | + | ± |
Documentation of avoidance of β-blocker withdrawal is a frequent tool by which “quality” of anesthesia services can be assessed by regulatory agencies.
ACE inhibitors prolong survival in patients with congestive heart failure or left ventricular dysfunction. Chronic aspirin therapy reduces coronary events in patients with CAD and prevents coronary and ischemic cerebral events in at-risk patients. Antiarrhythmic therapy in patients with complex ventricular ectopy who have significant CAD and left ventricular dysfunction should be guided by an electrophysiological study. Patients with inducible sustained ventricular tachycardia (VT) or ventricular fibrillation are candidates for an automatic internal cardioverter-defibrillator (ICD). Treatment of ventricular ectopy (with the exception of sustained VT) in patients with good ventricular function does not improve survival and may increase mortality. In contrast, ICDs have been shown to improve survival in patients with advanced cardiomyopathy (ejection fraction <30%), even in the absence of demonstrable arrhythmias.
Moderate to severe angina frequently requires combination therapy with two or all three classes of agents. Patients with ventricular dysfunction may not tolerate the combined negative inotropic effect of a β-blocker and a calcium channel blocker together; an ACE inhibitor is better tolerated and seems to improve survival. Similarly, the additive effect of a β-blocker and a calcium channel blocker on the AV node may precipitate heart block in susceptible patients.
The importance of ischemic heart disease—particularly a history of MI—as a risk factor for perioperative morbidity and mortality was discussed earlier in the chapter. Most studies confirm that perioperative outcome is related to disease severity, ventricular function, and the type of surgery to be undertaken.  Patients with extensive (three-vessel or left main) CAD, a recent history of MI, or ventricular dysfunction are at greatest risk of cardiac complications. As mentioned above, current guidelines recommend revascularization when such treatment would be indicated irrespective of the patient’s need for surgery.
Patients with extensive (three-vessel or left main) CAD, a recent history of MI, or ventricular dysfunction are at greatest risk of cardiac complications. As mentioned above, current guidelines recommend revascularization when such treatment would be indicated irrespective of the patient’s need for surgery.
Chronic stable (mild to moderate) angina does not seem to increase perioperative risk substantially. Similarly, a history of prior coronary artery bypass surgery or coronary angioplasty alone does not seem to substantially increase perioperative risk. In some studies, maintenance of chronic β-receptor blockers in the perioperative period has been shown to reduce perioperative mortality and the incidence of postoperative cardiovascular complications; however, other studies have shown an increase in stroke and death following preoperative introduction of β-blockers to “at risk” patients. Consequently, as with all drugs, the risks and benefits of initiating therapy with β-blockers in at risk patients must be considered. Like β-blockers, statins should be continued perioperatively in patients so routinely treated, as acute perioperative withdrawal of statins is associated with adverse outcomes. ACC/AHA guidelines suggest that β-blockers are useful in patients undergoing vascular surgery with evidence of ischemia on their evaluative workup (class I).
The history is of prime importance in patients with ischemic heart disease. Questions should encompass symptoms, current and past treatment, complications, and the results of previous evaluations. This information alone is usually enough to provide some estimate of disease severity and ventricular function.
The most important symptoms to elicit include chest pains, dyspnea, poor exercise tolerance, syncope, or near syncope. The relationship between symptoms and activity level should be established. Activity should be described in terms of everyday tasks, such as walking or climbing stairs. Patients may be relatively asymptomatic despite severe CAD if they have a sedentary lifestyle. Patients with diabetes are particularly prone to silent ischemia. The patient’s description of chest pains may suggest a major role for vasospasm (variable-threshold angina). Easy fatigability or shortness of breath suggests impaired ventricular function.
A history of unstable angina or MI should include the time of its occurrence and whether it was complicated by arrhythmias, conduction disturbances, or heart failure. Localization of the areas of ischemia is invaluable in deciding which electrocardiographic leads to monitor intraoperatively. Arrhythmias and conduction abnormalities are more common in patients with previous infarction and in those with poor ventricular function. This latter group of patients will often have ICDs.
Evaluation of patients with CAD is similar to that of patients with hypertension. Laboratory evaluation in patients who have a history compatible with recent unstable angina and are undergoing emergency procedures should include cardiac enzymes. Serum levels of cardiac-specific troponins, creatine kinase (MB isoenzyme), and lactate dehydrogenase (type 1 isoenzyme) are useful in excluding MI.
The baseline ECG is normal in 25% to 50% of patients with CAD but no prior MI. Electrocardiographic evidence of ischemia often becomes apparent only during chest pain. The most common baseline abnormalities are nonspecific ST-segment and T-wave changes. Prior infarction is often manifested by Q waves or loss of R waves in the leads closest to the infarct. First-degree AV block, bundle-branch block, or hemiblock may be present. Persistent ST-segment elevation following MI may be indicative of a left ventricular aneurysm. A long rate-corrected QT interval (QTc > 0.44 s) may reflect the underlying ischemia, drug toxicity (usually class Ia antiarrhythmic agents, antidepressants, or phenothiazines), electrolyte abnormalities (hypokalemia or hypomagnesemia), autonomic dysfunction, mitral valve prolapse, or, less commonly, a congenital abnormality. Patients with a long QT interval are at risk of developing ventricular arrhythmias—particularly polymorphic VT (torsade de pointes), which can lead to ventricular fibrillation. The long QT interval reflects nonuniform prolongation of ventricular repolarization and predisposes patients to reentry phenomena. In contrast to polymorphic ventricular arrhythmias with a normal QT interval, which respond to conventional antiarrhythmics, polymorphic tachyarrhythmias with a long QT interval generally respond best to pacing or magnesium salts. Patients with congenital prolongation generally respond to β-adrenergic blocking agents. Left stellate ganglion blockade is also effective and suggests that autonomic imbalance plays an important role in this group of patients.
The chest film can be used to exclude cardiomegaly or pulmonary vascular congestion secondary to ventricular dysfunction. Rarely, calcification of the coronaries, aorta, or the aortic valve may be seen on the chest radiograph; such is a more common finding on CT.
When used as screening tests for the general population, noninvasive stress tests have a low predictability in asymptomatic patients, but are sufficiently reliable in symptomatic patients with suspect lesions.  Holter monitoring, exercise electrocardiography, myocardial perfusion scans, and echocardiography are important in determining perioperative risk and the need for coronary angiography; however, these tests are indicated only if their outcome would alter patient care.
Holter monitoring, exercise electrocardiography, myocardial perfusion scans, and echocardiography are important in determining perioperative risk and the need for coronary angiography; however, these tests are indicated only if their outcome would alter patient care.
Current ACC/AHA guidelines recommend noninvasive stress testing in patients scheduled for noncardiac surgery with active cardiac conditions (class I). The current guidelines also suggest that there may be benefit of such testing in patients with three or more clinical risk factors and poor functional capacity (class IIa). Likewise, they suggest that noninvasive testing can be of some possible benefit in patients with one or two clinical risk factors undergoing intermediate risk or vascular surgery (class IIb). What they do not recommend is the indiscriminate use of noninvasive cardiac testing in patients with no risk factors undergoing intermediate-risk surgery. Consequently, indications for preoperative cardiac screening tests continue to narrow.
Continuous ambulatory electrocardiographic (Holter) monitoring is useful in evaluating arrhythmias, antiarrhythmic drug therapy, and severity and frequency of ischemic episodes. Silent (asymptomatic) ischemic episodes are frequently found in patients with CAD. Frequent ischemic episodes on preoperative Holter monitoring correlate well with intraoperative and postoperative ischemia. Holter monitoring has an excellent negative predictive value for postoperative cardiac complications.
The usefulness of this test is limited in patients with baseline ST-segment abnormalities and those who are unable to increase their heart rate (>85% of maximal predicted) because of fatigue, dyspnea, or drug therapy. Overall sensitivity is 65%, and specificity is 90%. The test is most sensitive (85%) in patients with three-vessel or left main CAD. Disease that is limited to the left circumflex artery may also be missed because ischemia in its distribution may not be evident on the standard surface ECG. A normal test does not necessarily exclude CAD, but suggests that severe disease is not likely. The degree of ST-segment depression, its severity and configuration, the time of onset in the test, and the time required for resolution are important findings. A myocardial ischemic response at low levels of exercise is associated with a significantly increased risk of perioperative complications and long-term cardiac events. Other significant findings include changes in blood pressure and the occurrence of arrhythmias. Exercise-induced ventricular ectopy frequently indicates severe CAD associated with ventricular dysfunction. The ischemia presumably leads to electrical instability in myocardial cells. Given that risk seems to be associated with the degree of myocardium potentially ischemic, testing often includes perfusion scans or echocardiographic assessments; however, in ambulatory patients, exercise ECG testing is useful because it estimates functional capacity and detects myocardial ischemia.
Myocardial perfusion imaging using thallium-201 or technetium-99m is used in evaluating patients who cannot exercise (eg, peripheral vascular disease) or who have underlying ECG abnormalities that preclude interpretation during exercise (eg, left bundle-branch block). If the patient cannot exercise, images are obtained before and after injection of an intravenous coronary dilator (eg, dipyridamole or adenosine) to produce a hyperemic response similar to exercise. Myocardial perfusion studies following exercise or injection of dipyridamole or adenosine have a high sensitivity, but only fairly good specificity for CAD. They are best for detecting two- or three-vessel disease. These scans can locate and quantitate areas of ischemia or scarring and differentiate between the two. Perfusion defects that fill in on the redistribution phase represent ischemia, not previous infarction. The negative predictive value of a normal perfusion scan is approximately 99%.
MRI, PET, and CT scans are increasingly being used to define coronary artery anatomy and determine myocardial viability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree