Key Concepts
 Clinical manifestations of bone cement implantation syndrome include hypoxia (increased pulmonary shunt), hypotension, arrhythmias (including heart block and sinus arrest), pulmonary hypertension (increased pulmonary vascular resistance), and decreased cardiac output.
Clinical manifestations of bone cement implantation syndrome include hypoxia (increased pulmonary shunt), hypotension, arrhythmias (including heart block and sinus arrest), pulmonary hypertension (increased pulmonary vascular resistance), and decreased cardiac output.
 Use of a pneumatic tourniquet on an extremity creates a bloodless field that greatly facilitates the surgery. However, tourniquets can produce potential problems of their own, including hemodynamic changes, pain, metabolic alterations, arterial thromboembolism, and pulmonary embolism.
Use of a pneumatic tourniquet on an extremity creates a bloodless field that greatly facilitates the surgery. However, tourniquets can produce potential problems of their own, including hemodynamic changes, pain, metabolic alterations, arterial thromboembolism, and pulmonary embolism.
 Fat embolism syndrome classically presents within 72 h following long-bone or pelvic fracture, with the triad of dyspnea, confusion, and petechiae.
Fat embolism syndrome classically presents within 72 h following long-bone or pelvic fracture, with the triad of dyspnea, confusion, and petechiae.
 Deep vein thrombosis and pulmonary embolism can cause morbidity and mortality following orthopedic operations on the pelvis and lower extremities.
Deep vein thrombosis and pulmonary embolism can cause morbidity and mortality following orthopedic operations on the pelvis and lower extremities.
 Neuraxial anesthesia alone or combined with general anesthesia may reduce thromboembolic complications by several mechanisms, including sympathectomy-induced increases in lower extremity venous blood flow, systemic antiinflammatory effects of local anesthetics, decreased platelet reactivity, attenuated postoperative increase in factor VIII and von Willebrand factor, attenuated postoperative decrease in antithrombin III, and alterations in stress hormone release.
Neuraxial anesthesia alone or combined with general anesthesia may reduce thromboembolic complications by several mechanisms, including sympathectomy-induced increases in lower extremity venous blood flow, systemic antiinflammatory effects of local anesthetics, decreased platelet reactivity, attenuated postoperative increase in factor VIII and von Willebrand factor, attenuated postoperative decrease in antithrombin III, and alterations in stress hormone release.
 For patients receiving prophylactic low-molecular-weight heparin once daily, neuraxial techniques may be performed (or neuraxial catheters removed) 10-12 h after the previous dose, with a 4-h delay before administering the next dose.
For patients receiving prophylactic low-molecular-weight heparin once daily, neuraxial techniques may be performed (or neuraxial catheters removed) 10-12 h after the previous dose, with a 4-h delay before administering the next dose.
 Flexion and extension lateral radiographs of the cervical spine should be obtained preoperatively in patients with rheumatoid arthritis severe enough to require steroids, immune therapy, or methotrexate. If atlantoaxial instability is present, intubation should be performed with inline stabilization utilizing video or fiberoptic laryngoscopy.
Flexion and extension lateral radiographs of the cervical spine should be obtained preoperatively in patients with rheumatoid arthritis severe enough to require steroids, immune therapy, or methotrexate. If atlantoaxial instability is present, intubation should be performed with inline stabilization utilizing video or fiberoptic laryngoscopy.
 Effective communication between the anesthesiologist and surgeon is essential during bilateral hip arthroplasty. If major hemodynamic instability occurs during the first hip replacement procedure, the second arthroplasty should be postponed.
Effective communication between the anesthesiologist and surgeon is essential during bilateral hip arthroplasty. If major hemodynamic instability occurs during the first hip replacement procedure, the second arthroplasty should be postponed.
 Adjuvants such as opioids, clonidine, ketorolac, and neostigmine when added to local anesthetic solutions for intraarticular injection have been used in various combinations to extend the duration of analgesia following knee arthroscopy.
Adjuvants such as opioids, clonidine, ketorolac, and neostigmine when added to local anesthetic solutions for intraarticular injection have been used in various combinations to extend the duration of analgesia following knee arthroscopy.
 Effective postoperative analgesia facilitates early physical rehabilitation to maximize postoperative range of motion and prevent joint adhesions following knee replacement.
Effective postoperative analgesia facilitates early physical rehabilitation to maximize postoperative range of motion and prevent joint adhesions following knee replacement.
 The interscalene brachial plexus block using ultrasound or electrical stimulation is ideally suited for shoulder procedures. Even when general anesthesia is employed, an interscalene block can supplement anesthesia and provide effective postoperative analgesia.
The interscalene brachial plexus block using ultrasound or electrical stimulation is ideally suited for shoulder procedures. Even when general anesthesia is employed, an interscalene block can supplement anesthesia and provide effective postoperative analgesia.
Anesthesia for Orthopedic Surgery: Introduction
Orthopedic surgery challenges the anesthesia provider. The comorbidities of these patients vary widely based on age group. Patients may present as neonates with congenital limb deformities, as teenagers with sports-related injuries, as adults for procedures ranging from excision of minor soft-tissue mass to joint replacement, or at any age with bone cancer. This chapter focuses on perioperative care issues specific to patients undergoing common orthopedic surgical procedures. For example, patients with long bone fractures are predisposed to fat embolism syndrome. Patients are at increased risk for venous thromboembolism following pelvic, hip, and knee operations. Use of bone cement during arthroplasties can cause hemodynamic instability. Limb tourniquets limit blood loss but introduce additional risks.
Neuraxial and other regional anesthetic techniques play an important role in decreasing the incidence of perioperative thromboembolic complications, providing postoperative analgesia, and facilitating early rehabilitation and hospital discharge. Advances in surgical techniques, such as minimally invasive approaches to knee and hip replacement, are necessitating modifications in anesthetic and perioperative management to facilitate overnight or even same-day discharge of patients who formerly required days of hospitalization. It is impossible to cover the anesthetic implications of diverse orthopedic operations in one chapter; hence, the focus here on perioperative management considerations and strategies for the anesthetic management of patients undergoing select orthopedic surgical procedures. Anesthesia for surgery on the spine is discussed in Chapter 27.
Bone cement, polymethylmethacrylate, is frequently required for joint arthroplasties. The cement interdigitates within the interstices of cancellous bone and strongly binds the prosthetic device to the patient’s bone. Mixing polymerized methylmethacrylate powder with liquid methylmethacrylate monomer causes polymerization and cross-linking of the polymer chains. This exothermic reaction leads to hardening of the cement and expansion against the prosthetic components. The resultant intramedullary hypertension (>500 mm Hg) can cause embolization of fat, bone marrow, cement, and air into venous channels. Systemic absorption of residual methylmethacrylate monomer can produce vasodilation and a decrease in systemic vascular resistance. The release of tissue thromboplastin may trigger platelet aggregation, microthrombus formation in the lungs, and cardiovascular instability as a result of the circulation of vasoactive substances.
 The clinical manifestations of bone cement implantation syndrome include hypoxia (increased pulmonary shunt), hypotension, arrhythmias (including heart block and sinus arrest), pulmonary hypertension (increased pulmonary vascular resistance), and decreased cardiac output. Emboli most frequently occur during insertion of a femoral prosthesis for hip arthroplasty. Treatment strategies for this complication include increasing inspired oxygen concentration prior to cementing, monitoring to maintain euvolemia, creating a vent hole in the distal femur to relieve intramedullary pressure, performing high-pressure lavage of the femoral shaft to remove debris (potential microemboli), or using a femoral component that does not require cement.
The clinical manifestations of bone cement implantation syndrome include hypoxia (increased pulmonary shunt), hypotension, arrhythmias (including heart block and sinus arrest), pulmonary hypertension (increased pulmonary vascular resistance), and decreased cardiac output. Emboli most frequently occur during insertion of a femoral prosthesis for hip arthroplasty. Treatment strategies for this complication include increasing inspired oxygen concentration prior to cementing, monitoring to maintain euvolemia, creating a vent hole in the distal femur to relieve intramedullary pressure, performing high-pressure lavage of the femoral shaft to remove debris (potential microemboli), or using a femoral component that does not require cement.
Another source of concern related to the use of cement is the potential for gradual loosening of the prosthesis over time. Newer cementless implants are made of a porous material that allows natural bone to grow into them. Cementless prostheses generally last longer and may be advantageous for younger, active patients; however, healthy active bone formation is required and recovery may be longer compared to cemented joint replacements. Therefore, cemented prostheses are preferred for older (>80 years) and less active patients who often have osteoporosis or thin cortical bone. Practices continue to evolve regarding selection of cemented versus cementless implants, depending on the joint affected, patient, and surgical technique.
 Use of a pneumatic tourniquet on an extremity creates a bloodless field that greatly facilitates surgery. However, tourniquets can produce potential problems of their own, including hemodynamic changes, pain, metabolic alterations, arterial thromboembolism, and pulmonary embolism. Inflation pressure is usually set approximately 100 mm Hg higher than the patient’s baseline systolic blood pressure. Prolonged inflation (>2 h) routinely leads to transient muscle dysfunction from ischemia and may produce rhabdomyolysis or permanent peripheral nerve damage. Tourniquet inflation has also been associated with increases in body temperature in pediatric patients undergoing lower extremity surgery.
Use of a pneumatic tourniquet on an extremity creates a bloodless field that greatly facilitates surgery. However, tourniquets can produce potential problems of their own, including hemodynamic changes, pain, metabolic alterations, arterial thromboembolism, and pulmonary embolism. Inflation pressure is usually set approximately 100 mm Hg higher than the patient’s baseline systolic blood pressure. Prolonged inflation (>2 h) routinely leads to transient muscle dysfunction from ischemia and may produce rhabdomyolysis or permanent peripheral nerve damage. Tourniquet inflation has also been associated with increases in body temperature in pediatric patients undergoing lower extremity surgery.
Exsanguination of a lower extremity and tourniquet inflation cause a rapid shift of blood volume into the central circulation. Although not usually clinically important, bilateral lower extremity exsanguination can cause an increase in central venous pressure and arterial blood pressure that may not be well tolerated in patients with noncompliant ventricles and diastolic dysfunction.
Awake patients predictably experience tourniquet pain with inflation pressures of 100 mm Hg above systolic blood pressure for more than a few minutes. The mechanism and neural pathways for this severe aching and burning sensation defy precise explanation. Tourniquet pain gradually becomes so severe over time that patients may require substantial supplemental analgesia, if not general anesthesia, despite a regional block that is adequate for surgical anesthesia. Even during general anesthesia, stimulus from tourniquet compression often manifests as a gradually increasing mean arterial blood pressure beginning approximately 1 h after cuff inflation. Signs of progressive sympathetic activation include marked hypertension, tachycardia, and diaphoresis. The likelihood of tourniquet pain and its accompanying hypertension may be influenced by many factors, including anesthetic technique (regional anesthesia versus general anesthesia), extent of dermatomal spread of regional anesthetic block, choice of local anesthetic and dose (“intensity” of block), and supplementation with adjuvants either intravenously or in combination with local anesthetic solutions when applicable.
Cuff deflation invariably and immediately relieves tourniquet pain and associated hypertension. In fact, cuff deflation may be accompanied by a precipitous decrease in central venous and arterial blood pressure. Heart rate usually increases and core temperature decreases. Washout of accumulated metabolic wastes in the ischemic extremity increases partial pressure of carbon dioxide in arterial blood (Paco2), end-tidal carbon dioxide (Etco2), and serum lactate and potassium levels. These metabolic alterations can cause an increase in minute ventilation in the spontaneously breathing patient and, rarely, arrhythmias. Tourniquet-induced ischemia of a lower extremity may lead to the development of deep venous thrombosis. Transesophageal echocardiography can detect subclinical pulmonary embolism (miliary emboli in the right atrium and ventricle) following tourniquet deflation even in minor cases such as diagnostic knee arthroscopy. Rare episodes of massive pulmonary embolism during total knee arthroplasty have been reported during leg exsanguination, after tourniquet inflation, and following tourniquet deflation. Tourniquets have been safely used in patients with sickle cell disease, although particular attention should be paid to maintaining oxygenation, normocarbia or hypocarbia, hydration, and normothermia.
Some degree of fat embolism probably occurs with all long-bone fractures. Fat embolism syndrome is less frequent but potentially fatal (10-20% mortality).  It classically presents within 72 h following long-bone or pelvic fracture, with the triad of dyspnea, confusion, and petechiae. This syndrome can also be seen following cardiopulmonary resuscitation, parental feeding with lipid infusion, and liposuction. The most popular theory for its pathogenesis holds that fat globules are released by the disruption of fat cells in the fractured bone and enter the circulation through tears in medullary vessels. An alternative theory proposes that the fat globules are chylomicrons resulting from the aggregation of circulating free fatty acids caused by changes in fatty acid metabolism. Regardless of their source, the increased free fatty acid levels can have a toxic effect on the capillary-alveolar membrane leading to the release of vasoactive amines and prostaglandins and the development of acute respiratory distress syndrome (ARDS; see Chapter 57). Neurological manifestations (eg, agitation, confusion, stupor, or coma) are the probable result of capillary damage in the cerebral circulation and cerebral edema. These signs may be exacerbated by hypoxia.
It classically presents within 72 h following long-bone or pelvic fracture, with the triad of dyspnea, confusion, and petechiae. This syndrome can also be seen following cardiopulmonary resuscitation, parental feeding with lipid infusion, and liposuction. The most popular theory for its pathogenesis holds that fat globules are released by the disruption of fat cells in the fractured bone and enter the circulation through tears in medullary vessels. An alternative theory proposes that the fat globules are chylomicrons resulting from the aggregation of circulating free fatty acids caused by changes in fatty acid metabolism. Regardless of their source, the increased free fatty acid levels can have a toxic effect on the capillary-alveolar membrane leading to the release of vasoactive amines and prostaglandins and the development of acute respiratory distress syndrome (ARDS; see Chapter 57). Neurological manifestations (eg, agitation, confusion, stupor, or coma) are the probable result of capillary damage in the cerebral circulation and cerebral edema. These signs may be exacerbated by hypoxia.
The diagnosis of fat embolism syndrome is suggested by petechiae on the chest, upper extremities, axillae, and conjunctiva. Fat globules occasionally may be observed in the retina, urine, or sputum. Coagulation abnormalities such as thrombocytopenia or prolonged clotting times are occasionally present. Serum lipase activity may be elevated but does not predict disease severity. Pulmonary involvement typically progresses from mild hypoxia and a normal chest radiograph to severe hypoxia or respiratory failure with radiographic findings of diffuse pulmonary opacities. Most of the classic signs and symptoms of fat embolism syndrome occur 1-3 days after the precipitating event. During general anesthesia, signs may include a decline in Etco2 and arterial oxygen saturation and a rise in pulmonary artery pressures. Electrocardiography may show ischemic-appearing ST-segment changes and a pattern of right-sided heart strain.
Management is two-fold: preventative and supportive. Early stabilization of the fracture decreases the incidence of fat embolism syndrome and, in particular, reduces the risk of pulmonary complications. Supportive treatment consists of oxygen therapy with continuous positive airway pressure ventilation to prevent hypoxia and with specific ventilator strategies in the event of ARDS. Systemic hypotension will require appropriate pressor support, and vasodilators may aid the management of pulmonary hypertension. High-dose corticosteroid therapy is not supported by randomized clinical trials.
 Deep vein thrombosis (DVT) and pulmonary embolism (PE) can cause morbidity and mortality following orthopedic operations on the pelvis and lower extremities. Risk factors include obesity, age greater than 60 years, procedures lasting more than 30 min, use of a tourniquet, lower extremity fracture, and immobilization for more than 4 days. Patients at greatest risk include those undergoing hip surgery and knee replacement or major operations for lower extremity trauma. Such patients will experience DVT rates of 40-80% without prophylaxis. The incidence of clinically important PE following hip surgery in some studies is reported to be as high as 20%, whereas that of fatal PE may be 1-3%. Underlying pathophysiological mechanisms include venous stasis with hypercoagulable state due to localized and systemic inflammatory responses to surgery.
Deep vein thrombosis (DVT) and pulmonary embolism (PE) can cause morbidity and mortality following orthopedic operations on the pelvis and lower extremities. Risk factors include obesity, age greater than 60 years, procedures lasting more than 30 min, use of a tourniquet, lower extremity fracture, and immobilization for more than 4 days. Patients at greatest risk include those undergoing hip surgery and knee replacement or major operations for lower extremity trauma. Such patients will experience DVT rates of 40-80% without prophylaxis. The incidence of clinically important PE following hip surgery in some studies is reported to be as high as 20%, whereas that of fatal PE may be 1-3%. Underlying pathophysiological mechanisms include venous stasis with hypercoagulable state due to localized and systemic inflammatory responses to surgery.
Pharmacological prophylaxis and the routine use of mechanical devices such as intermittent pneumatic compression (IPC) have been shown to decrease the incidence of DVT and PE. While mechanical thromboprophylaxis should be considered for every patient, the use of pharmacological anticoagulants must be balanced against the risk of major bleeding. For patients at increased risk for DVT but having “normal” bleeding risk, low-dose subcutaneous unfractionated heparin (LUFH), warfarin, or low-molecular-weight heparin (LMWH) may be employed in addition to mechanical prophylaxis. Patients at significantly increased risk of bleeding may be managed with mechanical prophylaxis alone until bleeding risk decreases. In general, anticoagulants are started the day of surgery in patients without indwelling epidural catheters. Warfarin may be started the night before surgery depending on the particular orthopedic surgeon’s routine.
 Neuraxial anesthesia alone or combined with general anesthesia may reduce thromboembolic complications by several mechanisms. These include sympathectomy-induced increases in lower extremity venous blood flow, systemic antiinflammatory effects of local anesthetics, decreased platelet reactivity, attenuated postoperative increases in factor VIII and von Willebrand factor, attenuated postoperative decreases in antithrombin III, and alterations in stress hormone release.
Neuraxial anesthesia alone or combined with general anesthesia may reduce thromboembolic complications by several mechanisms. These include sympathectomy-induced increases in lower extremity venous blood flow, systemic antiinflammatory effects of local anesthetics, decreased platelet reactivity, attenuated postoperative increases in factor VIII and von Willebrand factor, attenuated postoperative decreases in antithrombin III, and alterations in stress hormone release.
According to the Third Edition of the American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines on regional anesthesia and anticoagulation, patients currently receiving antiplatelet agents (eg, ticlopidine, clopidogrel, and intravenous glycoprotein IIb/IIIa inhibitors), thrombolytics, fondaparinux, direct thrombin inhibitors, or therapeutic regimens of LMWH present an unacceptable risk for spinal or epidural hematoma following neuraxial anesthesia. Performance of neuraxial block (or removal of a neuraxial catheter) is not contraindicated with subcutaneous LUFH when the total daily dose is 10,000 units or less; there are no data on the safety of neuraxial anesthesia when larger doses are given.  For patients receiving prophylactic LMWH, the guidelines vary based on regimen. With once-daily dosing, neuraxial techniques may be performed (or neuraxial catheters removed) 10-12 h after the previous dose, with a 4-h delay before administering the next dose. With twice-daily dosing, neuraxial catheters should not be left in situ and should be removed 2 h before the first dose of LMWH. Patients on warfarin therapy should not receive a neuraxial block unless the international normalized ratio (INR) is normal, and catheters should be removed when the INR is 1.5 or lower. The Third Edition of the guidelines also suggests that these recommendations be applied to deep peripheral nerve and plexus blocks and catheters (see Suggested Reading). Revisions to these guidelines occur regularly.
For patients receiving prophylactic LMWH, the guidelines vary based on regimen. With once-daily dosing, neuraxial techniques may be performed (or neuraxial catheters removed) 10-12 h after the previous dose, with a 4-h delay before administering the next dose. With twice-daily dosing, neuraxial catheters should not be left in situ and should be removed 2 h before the first dose of LMWH. Patients on warfarin therapy should not receive a neuraxial block unless the international normalized ratio (INR) is normal, and catheters should be removed when the INR is 1.5 or lower. The Third Edition of the guidelines also suggests that these recommendations be applied to deep peripheral nerve and plexus blocks and catheters (see Suggested Reading). Revisions to these guidelines occur regularly.
Hip Surgery
Common hip procedures performed in adults include repair of hip fracture, total hip arthroplasty, and closed reduction of hip dislocation.
Most patients presenting for hip fractures are frail and elderly. An occasional young patient will have sustained major trauma to the femur or pelvis. Studies have reported mortality rates following hip fracture of up to 10% during the initial hospitalization and over 25% within 1 year. Many of these patients have concomitant diseases such as coronary artery disease, cerebrovascular disease, chronic obstructive pulmonary disease, or diabetes.
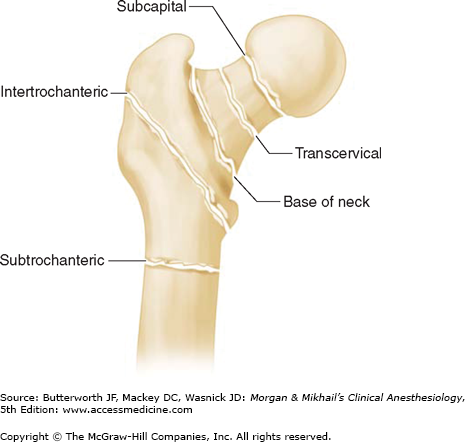
Patients presenting with hip fractures are frequently dehydrated from inadequate oral intake. Depending on the site of the hip fracture, occult blood loss may be significant, further compromising intravascular volume. In general, intracapsular (subcapital, transcervical) fractures are associated with less blood loss than extracapsular (base of the femoral neck, intertrochanteric, subtrochanteric) fractures (Figure 38-1). A normal or borderline-low preoperative hematocrit may be deceiving when hemoconcentration masks occult blood loss.
Another characteristic of hip fracture patients is the frequent presence of preoperative hypoxia that may, at least in part, be due to fat embolism; other factors can include bibasilar atelectasis from immobility, pulmonary congestion (and effusion) from congestive heart failure, or consolidation due to infection.