Figure 7.1
Pulmonary bullae
Spontaneous nontraumatic pneumothorax is a clinical entity, usually associated with underlying pulmonary pathology (Table. 7.1). Primary spontaneous pneumothorax is due to rupture of pulmonary bullae (Fig. 7.1), and risk factors include tobacco smoking, tall stature, and age between 15 and 35 or over 55 years [3]. Secondary spontaneous pneumothorax is associated with chronic obstructive pulmonary disease (COPD), cystic fibrosis, asthma, and lung cancer.
Table 7.1
Main causes of spontaneous nontraumatic hemothorax
Neoplasia (primary or metastatic) |
Bullous emphysema |
Tuberculosis |
Anticoagulation complications |
Necrotizing infections |
Pulmonary arteriovenous fistula |
Pulmonary embolism with infarction |
Hereditary hemorrhagic telangiectasia |
7.1.2 Simple (Nontension) Pneumothorax
Simple pneumothorax results in decreased breath sounds on the affected side as well as hyper-resonance on percussion. Depending on the size of the pneumothorax, as well as on the noise in the examination environment, a simple pneumothorax can be difficult to be clinically detected [4]. In this case, an erect chest X-ray in expiration can establish the diagnosis. It is important to be alert of the fact that general anesthesia and positive pressure ventilation can result in the increase of the size of the pneumothorax, even leading to the creation of a tension pneumothorax, making the insertion of an intercostal drain obligatory as a preemptive step. Small closed pneumothoraces (less than 15 %) can be treated by oxygen administration and clinical observation, coupled with follow-up X-rays, as there is a good chance that this will lead to its resolution. Generally speaking, apart from the previous case, all pneumothoraces are dealt with by intercostal drain insertion.
7.1.3 Tension Pneumothorax
Tension pneumothorax is a life-threatening condition and develops when a “one-way valve” air leak results in accumulation of air under tension in the pleural cavity, the air originating from the lung, or through a chest wall defect. Particularly in the case of the latter, if its size is approximately the 2/3 of the diameter of trachea, the air will preferentially pass through the chest wall defect with each respiratory effort, as the air tends to follow the path of least resistance [5].
As the air is entrapped in the pleural cavity without means of escape, it results in intrapleural pressure increase with every breath, leading to collapse of the ipsilateral lung. There is displacement of the mediastinum to the opposite side with ipsilateral increase of the intercostal spaces and depression of the diaphragm. The combination of the above leads to marked decrease in venous return, causing reduction of cardiac output. Any simple pneumothorax can be converted to tension pneumothorax irrespective of its cause; therefore, the clinician must be vigorously alert. This can occur particularly if a patient with a simple pneumothorax is on positive pressure ventilation.
The diagnosis of tension pneumothorax should be clinical as the condition is life threatening and the clinical symptoms and signs are profoundly obvious. The patient presents with chest pain – frequently related to concomitant rib fractures – air hunger and respiratory distress. There is deviation of the trachea to the opposite side, distention of the neck veins, tympanic sound on percussion, and absence of breath sounds on auscultation. There is tachycardia, sometimes related with hypotension. Cyanosis is a late sign.
The treatment is rapid insertion of a large caliber needle into the second intercostal space in the midclavicular line of the affected side. This should be followed by the insertion of an intercostal drain and the simultaneous occlusion on the thoracic wall opening usually with stitches [6]. In the presence of an open pneumothorax, secondary to a large defect of the chest wall, this defect must be managed by the application of a “three side dressing.” This should be square shaped, sterile occlusive dressing, large enough to overlap the edges of the wound, and should be taped on the three of its four sides in order to provide a flutter-type valve effect. On inspiration, the dressing occludes the wound preventing air from entering, while on expiration, the nontaped side of the dressing allows air to escape from the pleural cavity [2]. “Bubbling” at the intercostal drain collecting canister is an indication of air leak related to bronchial injury (Fig. 7.2) [7]. This may require an operative management of the pneumothorax.


Figure 7.2
“Bubbling” inside the intercostal drain collection canister: indication of major air leak probably related to bronchial injury
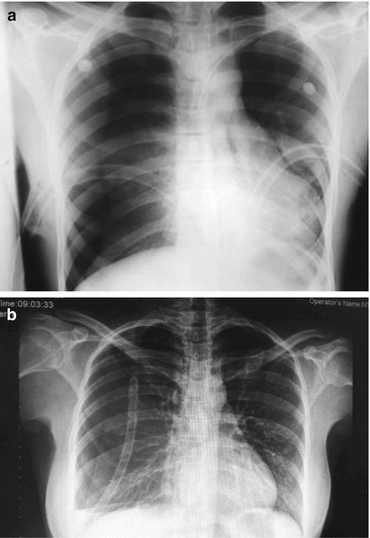
7.2 Hemothorax
7.2.1 Simple Hemothorax
This is characterized by collection of less than 1500 ml of blood in the pleural cavity, and in most cases, it is related to lung laceration or injury of an intercostal vessel. It may infrequently take place in nontraumatic cases, particularly in pleural infiltration by malignancy, complication of infectious disease (e.g., tuberculosis), erosion of a vessel, or rupture of an aneurysm.
Clinical examination will reveal decreased breath sound on auscultation, and dullness on percussion particularly on the examination of the posterior lower hemithorax, with the patient in the sitting-up position. An erect chest X-ray will show obliteration of the costophrenic angle, formed by the accumulation of blood (if it is less than 500 cc, it could well not show in the erect X-ray), or by an air-fluid level in the presence of a concomitant pneumothorax. The supine chest X-ray will show a homogeneous haziness of the hemithorax that is caused by the creation of a film of blood of variable “thickness” (relevant to the volume of the blood retained) that lies at the posterior aspect of the affected pleural cavity. The treatment is the insertion of an intercostal drain, which should be directed downwards and backwards (Fig. 7.3a proper positioning, Fig. 7.3b improper positioning) so that the drainage of the hemothorax would be effective irrespective if the patient is sitting up or lies flat in his bed [8]. Taking into consideration the fact that many times hemothorax coincides with pneumothorax, this is the ideal positioning of the intercostal drain, as the pneumothorax will, in any case, be drained from the pleural cavity irrespective of the positioning of the intercostal drain as the air will be pumped out the pleural cavity by the lung expansion during inspiration. In contradiction, this is not always happening with a hemothorax, which is usually retained at the lower posterior part of the pleural cavity, where due to gravity, it is mostly accumulated [9]. Insertion of another drain in the presence of retained hemothorax is acceptable. It is important to make sure that there is no retained hemothorax after the drains stop working or after they get removed. A chest X-ray at those cases is imperative. If there is retained hemothorax that cannot be drained with a further insertion with an intercostal drain, a video-assisted thoracoscopy (VATS) should be considered. If a retained hemothorax is not drained, there is a possibility of development of an empyema. It is recommended that VATS should take place at an early stage preferably after the fifth post injury day. Otherwise, evacuation of the retained clot can be technically difficult. Instillation of streptokinase for liquidification has been suggested but has not found wide application.