
EXTREMITY VASCULAR INJURIES
HISTORY
Vascular injuries in the extremities have been reported since antiquity. Aulus Cornelius Celsus (25 BC-50 AD) first described that hemorrhage from such injuries could be treated by placing “ligatures… above and below the wounded part.”1 For the next 18 centuries, control of hemorrhage dominated all medical writings on extremity vascular injuries. Repair of an injured brachial artery was first performed in 1759 and reported by Hallowell in 1762.2 The first repair of an injured artery in an extremity (the common femoral artery) in the United States was reported by John B. Murphy in Chicago in 1897.3 Essentially all the modern suture techniques of vascular repair in the extremities were described by Charles C. Guthrie and Alexis Carrel during and after their collaboration in the Hull Physiological Laboratory at the University of Chicago from 1905 to 1906.4,5
Surgeons in military conflicts who made considerable contributions to the repair of vessels in the extremities over the past century have included the following: Sir George Henry Makins (1853–1933) in World War I; Michael E. DeBakey (1908–2008), Daniel C. Elkin (1883–1958), and Harris B. Shumacker, Jr. (1908–2009) in World War II; Frank C. Spencer (1925-) and Carl W. Hughes (1914-) in the Korean War; and Norman M. Rich (1934-) during the Vietnam War.6 Recognizing that other forms of therapy are now used on occasion to treat vascular injuries in the extremities, the early laboratory description of stents/stent grafts was by the late Charles T. Dotter in 1969.7
EPIDEMIOLOGY
When considering all injuries admitted to military or civilian hospitals prior to the current conflicts in Iraq and Afghanistan, vascular injuries are uncommon. Frykberg and Schinco8 noted that the incidence of vascular injuries in four military reports (1913; 1946; 1952; 1978) ranged from 0.4% to 2%, while two civilian reports (1991; 1992) had incidences ranging from 0.8% to 3.7%. Of interest, the distribution of vascular injuries varies widely in the United States. The incidence of vascular injuries in the extremities as a percentage of all vascular injuries has ranged from 38% to 50% in centers dealing with a significant number of gunshot wounds to >75% in centers dealing primarily with blunt trauma.9
TYPES OF INJURIES/PATHOPHYSIOLOGY
There are seven recognized types of vascular injuries: (1) intimal injuries (flaps, defects, or subintimal hematomas); (2) partial wall defects (intima and media) presenting as traumatic “true” aneurysms; (3) complete wall defects presenting as traumatic “false” aneurysms or hemorrhage; (5) complete transections; (6) arteriovenous fistulas; and (7) spasm. Intimal injuries and partial wall defects continue to be most commonly associated with blunt trauma (i.e., blunt posterior dislocation of the knee joint with secondary thrombosis of the popliteal artery). In contrast, complete wall defects, near-complete or complete transections, and arteriovenous fistulas are most commonly associated with penetrating wounds. Arterial spasm can occur after either blunt or penetrating trauma to an extremity and is frequently noted distal to a proximal arterial injury that is occlusive.
PROGNOSTIC FACTORS
The age of the patient is a major factor in the success of peripheral arterial repair. With most peripheral arterial injuries occurring in patients under the age of 35 years, repairs are straightforward as vessels are soft, mobile, and without appreciable atherosclerosis. These characteristics allow for easy debridement and primary repair or end-to-anastomosis without the insertion of substitute vascular conduits in many patients. On the other hand, young patients do not have as many collateral vessels as might be seen in an older patient with slow atherosclerotic occlusion of the superficial femoral artery at Hunter’s canal. For this reason, the superficial femoral artery, popliteal artery, and tibioperoneal trunk should be regarded as “end” arteries in which acute occlusion is a threat to the viability of the ipsilateral leg or foot.
Shock secondary to the injury to the extremity or to other injuries has many adverse effects on a subsequent arterial or venous repair. The “viscerocutaneous vasoconstriction” that is associated with hypovolemic shock decreases whatever arterial inflow that remains through an injured artery or any regional collaterals and increases distal ischemia. With decreased distal flow, in situ thrombosis of capillaries, arterioles, or even named arteries may occur in the lower leg. Finally, ischemia followed by restoration of arterial inflow is the classical sequence of the “ischemia-reperfusion” syndrome.10 This syndrome increases edema of ischemic tissues distal to an arterial injury and may cause a compartment syndrome in the leg or forearm after arterial repair.11
The magnitude of a peripheral arterial or venous injury obviously impacts the success of a repair. When segmental resection of an injured vessel and insertion of an interposition graft, particularly a small Teflon one, are necessary, long-term patency is decreased.12
The impact of delay of an arterial repair on the success of salvaging an injured extremity is well known. The original description of the “6-hour window” to complete a major arterial repair in an injured extremity in dogs was described in 1949.13 Of interest, the 6-hour window continues to be valid in humans with major arterial injuries in extremities based on the time of onset of neural necrosis and myonecrosis when “cold ischemia” (no collateral flow) is present.14
The presence of an associated venous injury has a major impact on outcome. The need to simultaneously clamp an injured major artery and vein in a lower extremity increases the likelihood that a postrepair distal compartment syndrome will occur.11 In addition, ligation of a major vein in the lower extremity, such as the common femoral vein, had a significant adverse effect on femoral arterial inflow in one laboratory study.15 Finally, the presence of multiple associated injuries to soft tissue, tendons, bones, and nerves in an extremity—the so-called “mangled extremity”—will result in amputation rates of 28%-78% when the major artery is occluded or transected.16
Physical Examination
A patient with “hard” signs of a peripheral arterial injury (external hemorrhage, pulsatile hematoma, decrease in or loss of pulse and distal ischemic signs or palpable thrill/audible bruit) should be moved to the operating room immediately with several exceptions.17 It is appropriate to realign any displaced fracture with traction or a splint or to reduce any dislocated joint in the pulseless extremity to see if distal pulses return. In patients who are pulseless at the ankle or wrist and there is a shotgun wound to the extremity with wide dispersal of pellets or blunt trauma with fractures at several levels, some type of imaging study will be useful in localizing the site of injury. The final exception would include a patient with multiple injuries in whom there is a need to rule out life-threatening injuries to the brain, thorax, or abdomen using CT before moving the patient to the operating room.
Most patients present with “soft” signs of an arterial injury (history of arterial bleeding, proximity of extremity injury to a named artery, nonpulsatile hematoma, or injury to adjacent nerve).18 These patients still have an arterial pulse at the ankle or wrist on physical examination or with use of the Doppler device. Depending on which soft sign or combination of soft signs is present, the incidence of arterial injuries in such patients ranges from 3% to 25%.–1921 Most but not all, of these arterial injuries can be managed without surgery because they are small and, by definition, allow for continuing distal perfusion. In some centers, serial physical examinations alone are used to monitor distal pulses, and no CT—arteriogram or conventional arteriogram—is performed to document the magnitude of a possible arterial injury. This approach has been safe and accurate in asymptomatic patients with penetrating wounds to an extremity in proximity to a major artery.20,22 Its accuracy is similar in higher kinetic energy injuries associated with blunt fractures or dislocations, particularly dislocations of the knee.23 Observation is appropriate only with complete and continuing out-of-hospital follow-up.19,20 When there is concern about a distal pulse deficit, inability to properly examine for distal arterial pulses, or a combination of soft signs of an arterial injury in an extremity, CT, conventional, or surgeon-performed arteriography or a duplex ultrasound should be performed.
Based on the discussion above, it is obvious that a comprehensive physical examination of the injured extremity is the prime diagnostic maneuver in patients with possible peripheral vascular injuries. Relying on physical examination, particularly in patients with “soft” signs of an arterial injury in an extremity, has decreased considerably the number of imaging studies performed in the past 20 years.
Arterial Pressure Index
An adjunct to the physical examination is the measurement of the arterial pressure index (API). The API is defined as the Doppler systolic pressure in the injured extremity divided by that in an uninjured extremity.–2426 In a study by Lynch and Johansen25 in which clinical outcome was the standard, an API lower than 0.90 had a sensitivity of 95%, specificity of 97.5%, and accuracy of 97% in predicting an arterial injury. An alternative when both lower extremities are injured is to use the ankle brachial index, which uses brachial artery pressure as the denominator.
When the patient has an API < 0.9, the current standard of care is to perform an imaging study on the injured extremity.18,24–26
Imaging Studies
Multidetector computed tomography arteriography (MDCTA) is rapidly replacing conventional radiology suite arteriography or surgeon-performed arteriography in the emergency center or operating room.27,28 It can be performed rapidly with a reasonable amount of contrast and accuracy approaching conventional arteriography. When MDCTA is not available or the result is equivocal and the patient is hemodynamically stable, percutaneous intraarterial digital subtraction arteriography performed in a radiology suite by the interventional radiologist is the most commonly used invasive diagnostic technique. Of interest, this technique can now be performed in the trauma room with a mobile device.29 Multiple sequential views of areas of suspected arterial injury can be obtained at differing intervals after injection of limited amounts of dye. The accuracy of this multiple-view technique has been demonstrated in many studies, although false-negative results have occurred. The disadvantages of the technique are the delays in diagnosis when on-call technicians must return to the hospital, the cost of modern equipment, and the distortion of images when metallic fragments are present (e.g., shotgun wound).16
When the patient is hemodynamically unstable or has multiple injuries, a rapid one- or two-shot surgeon-performed percutaneous arteriogram can be performed in the emergency center or operating room.30,31 A thin-walled 18-gauge Cournand-style disposable needle is inserted either proximal to the area of suspected injury (e.g., in the common femoral artery for evaluation of the superficial femoral artery) or distal to it (e.g., in retrograde evaluation of axillary or subclavian arteries above a blood pressure cuff inflated to 300 mm Hg). Rapid hand injection of 35 mL of 60% diatrizoate meglumine dye is performed, and an anteroposterior radiographic view is taken or fluoroscopy is utilized. The timing for exposure of an x-ray film of the patient’s extremity depends on which artery is to be evaluated. Proper evaluation of the tibial and peroneal arteries in the patient with a complex fracture of the tibia mandates that exposure not take place until 4–5 seconds after the injection of dye into the common femoral artery. The plane of the film is often changed before the second injection to examine the area in question more thoroughly. False-negative and false-positive results are rare when the technique is performed on a daily basis by experienced practitioners. If a patient has severe combined intracranial or truncal trauma and possible peripheral arterial lesions, life-threatening injuries should be treated first, followed by percutaneous intraoperative arteriography of the involved extremity.16
Duplex ultrasonography, a combination of real-time B-mode ultrasound imaging and pulsed Doppler flow detection, has been used extensively to evaluate patients with suspected peripheral vascular injuries. Accuracy has ranged from 96% to 100% in several studies.–3234 The major disadvantage is the need for the study to be performed and interpreted by an experienced vascular surgeon or registered vascular technologist.
MANAGEMENT IN THE EMERGENCY CENTER
Hemorrhage resulting from a peripheral vascular injury is controlled by direct compression with a finger or compression dressing, compression at a “pressure point” or the application of a blood pressure cuff or tourniquet just proximal to the area of injury. Once hemorrhage is under temporary control in the patient with multisystem blunt trauma, a decision is made on which diagnostic imaging studies are necessary before transfer to the operating room.
When a peripheral arterial occlusion is associated with a delay in treatment or distal “cold ischemia” (inadequate or no collateral flow), the administration of intravenous heparin (100 U/kg) in the emergency center is appropriate. Contraindications include near-exsanguination from other injuries (coagulopathy may occur), a traumatic brain injury, a traumatic false aneurysm of the descending thoracic aorta or any other truncal artery, or CT documentation of an injury to a solid organ in the abdomen. Hemodynamically stable patients with isolated injuries to the extremities and “soft” signs undergo diagnostic evaluation and/or observation as described previously.
NONOPERATIVE MANAGEMENT/STENTS/EMBOLIZATION
Nonoperative Management
Nonocclusive arterial injuries including intimal defects or flaps, subintimal or intramural hematomas, spasm, and small pseudoaneurysms that are nonobstructing are present on imaging in some patients with “soft” signs on presentation. On follow-up arteriograms, 87%-95% of these lesions have healed without any surgical or radiologic intervention.35,36 Early arteriographic follow-up is necessary in patients who develop new symptoms while being observed. Careful follow-up of the injured patient and the extremity in an outpatient setting for a period of 2 months is mandatory.
Endovascular Stents
Currently, there is limited experience with the use of stents or stent grafts to treat peripheral arterial injuries.37,38 This is a reflection of the excellent results obtained with operative management of these injuries for the past 60 years.8,12,16 Therefore, the use of endovascular stents or stent grafts for peripheral vascular injuries should be limited to institutions in which there are approved protocols with plans for long-term follow-up of patients.18
Therapeutic Embolization by Interventional Radiology
Extravasation, a pseudoaneurysm, and arteriovenous fistula in the profunda femoris artery, one of the arteries in the leg or one of the branches of a major peripheral artery can be managed by embolization by an interventional radiologist. The ideal patient for such an intervention would be one with multisystem injuries, closed fractures, or late diagnosis of a traumatic aneurysm following orthopedic reconstruction.36
OPERATIVE MANAGEMENT
Preparation/Draping
Extensive preparation and draping of the skin is necessary. For a vascular repair in the upper extremity, this includes the chin to umbilicus and contralateral nipple to ipsilateral fingernails as well as one entire lower extremity to the toenails. In the lower extremity, preparation and draping would be from nipples to bilateral toenails. The hand or foot is then placed in a sterile plastic bag to allow for easy palpation of pulses after the arterial repair has been performed. Another option is to cover the entire extremity with an orthopaedic stockinette.
Preliminary or Concurrent Fasciotomy
Some patients have symptoms and signs of a compartment syndrome in the upper or lower extremity related to the peripheral vascular injury when first evaluated in the emergency center. For example, a patient with occlusion of the popliteal artery or a large hematoma in the anterior compartment of the leg might present with the following: (1) hypesthesia in the dorsal first web space; (2) weakness of toe extension and foot dorsiflexion; and (3) pain on passive toe flexion and foot plantar flexion.11 A measurement of compartment pressure is appropriate when symptoms and signs are suggestive, but unclear, or when there does not appear to be excessive swelling of the compartment. If there is physical evidence or a compartment pressure documenting the presence of a compartment syndrome distal to an arterial injury, a preliminary or concurrent (with the arterial repair) fasciotomy is indicated.39
Incisions
A longitudinal incision is made over the area of the peripheral vascular injury. Examples would include the medial arm incision in the biceps–triceps groove to expose the brachial artery or the anteromedial thigh incision to expose the superficial femoral artery beyond its origin in the groin. When the area of injury is in proximity to a joint, a gently curved incision is made to prevent a postoperative scar contracture. Examples would include the following: (1) axillobrachial “S” over an injury to the distal axillary/proximal brachial vessels (Fig. 31.1); (2) medial-to-lateral “S” over the antecubital area of the upper extremity to expose the distal brachial artery and its bifurcation; and (3) obtuse incision over the medial knee joint to expose the entire popliteal artery.
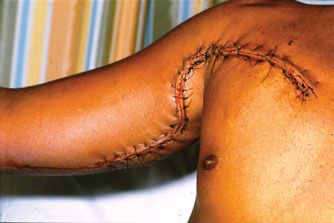
FIGURE 31.1. Standard incision for exposure of entire axillary and brachial arteries. (Reprinted from Feliciano DV. Peripheral vasculature. In: Britt LD, Trunkey DD, Feliciano DV, eds. Acute Care Surgery. Principles and Practice. New York: Springer;2007: 661, with permission from Springer.)
Proximal and Distal Vascular Control
Once the skin incision is made, it is deepened proximally and distally around the area of the presumed vascular injury to allow for vascular control. In other words, the part of the incision over the area of the presumed vascular injury is only deepened when proximal and distal vascular control has been attained with the use of small DeBakey vascular clamps, bulldog clamps, or vessel loops.
When hemorrhage cannot be controlled or a large hematoma is present, it is appropriate to enter the area of injury and rapidly apply vascular clamps directly around the perforation in the peripheral artery and/or vein. Adequate suction devices and appropriate retractors are mandatory to limit blood loss during this approach, but it allows for quicker vascular control in experienced hands.
Temporary Intraluminal Vascular Shunts
Once proximal and distal vascular control has been attained, there are two clinical scenarios in which insertion of a temporary intraluminal vascular shunt rather than repair or ligation is appropriate. The first is near-exsanguination with secondary hypothermia, a metabolic acidosis, or a coagulopathy likely to occur during resuscitation and operation. Rather than follow the classical dictum of “life over limb” and perform a ligation of an injured major artery of vein that may lead to an ultimate amputation, a shunt is inserted into the injured vessel. Once damage control resuscitation in the intensive care unit has reversed the “metabolic failure” of near-exsanguination, the patient is returned to the operating room for removal of the shunt and vascular repair.40,41
The second scenario is when a “mangled extremity” has resulted from a high kinetic injury impact (i.e., car bumper, motorcycle crash, penetrating wound from a military rifle) (Fig. 31.2). As previously noted, the combination of injuries to artery, bone, soft tissue, muscles/tendons, and peripheral nerves has resulted in ultimate amputation rates of 42%-78% in older series.42,43 There are, however, patients without all the components of injury listed who will be candidates for limb salvage instead of amputation. These patients have an intact posterior tibial nerve in the lower extremity or a crush injury with a limited warm ischemia time (i.e., <6 hours) in the upper or lower extremity. If limb salvage is to be considered or if the patient and family object to an immediate amputation, a shunt is inserted into the injured vessel.

FIGURE 31.2. A 14-F “carotid artery” shunt in the popliteal artery and a 24-F thoracostomy tube shunt in the popliteal vein in a railroad worker with a crush injury to the distal thigh. (Reprinted from Feliciano DV. Peripheral vasculature. In: Britt LD, Trunkey DD, Feliciano DV, eds. Acute Care Surgery. Principles and Practice. New York, NY: Springer; 2007: 670, with permission from Springer.)
The technique for insertion of a temporary intraluminal shunt is quite simple. For peripheral arterial injuries, a No. 14-Fr. or smaller rigid carotid artery-type shunt is cut to a length approximating the distance between the two ends of the debrided vessel plus another 3–4 cm. A 2-0 silk tie is placed around the middle of the shunt as a marker, a hemostat is applied to the same location, and the shunt is inserted into the proximal arterial stump for 1.5–2.0 cm. Another 2-0 silk tie is placed around the end of the artery containing the short segment of shunt and tied tight to compress the artery down onto the shunt. Proximal arterial control is released, the hemostat around the middle of the shunt is removed, and flow through the shunt is verified. The hemostat is placed on the middle of the shunt again, and the distal end of the shunt is directed into the distal end of the artery for 1.5–2.0 cm. Another 2-0 silk tie is placed to compress the distal artery around the distal end of the shunt, and the hemostat on the shunt is removed. Arterial pulsations in the distal extremity are then verified by physical examination or use of a Doppler-device. When a large peripheral vein is to be shunted rather than ligated, thoracostomy tubes in the No. 16–24 Fr. size range are used rather than the smaller arterial shunts described above.
The largest possible shunt that can be inserted in either a peripheral artery or vein is chosen. With use of a large shunt, postoperative anticoagulation is neither necessary nor appropriate in patients with multiple injuries or with substantial defects in soft tissues as might be seen with a mangled extremity. Thrombosis of an arterial shunt occurs when too small or too long a shunt is inserted, when a major adjacent vein is ligated rather than shunted, or when a delay in treatment has allowed for distal in situ thrombosis of multiple small arteries or veins in the leg or forearm. Thrombosis of an arterial shunt is an ominous predictor in that many such patients will eventually require amputation. This is particularly true when there is a delay in recognition of the thrombosis in the intensive care unit.
The longest dwell time for an arterial intraluminal shunt in a recent large civilian series was 52 hours,41 but there is one report of a patient with a 10-day dwell time in the right axillary artery without anticoagulation.44 Once the patient is stable or a decision has been reached that limb salvage is appropriate, the patient is returned to the operating room for removal of the shunt. The prior segmental resection of the injured vessel and the placement of crushing 2-0 silk ties on the remaining ends to hold the shunt in place mandate that an interposition graft will be inserted into the injured vessel. Therefore, an appropriate length of saphenous vein from the thigh or ankle of an uninjured lower extremity is removed in the usual fashion prior to removal of the arterial shunt. Should there be a large soft tissue defect over the arterial defect, a much longer segment of saphenous vein will have to be removed so that an extra-anatomic bypass (to be discussed) can be performed.44,45 When a large peripheral vein such as the axillary, common femoral, or superficial femoral is to be repaired (to be discussed), no retrieval of the saphenous vein will be necessary unless the surgeon is committed to constructing a panel or spiral vein graft. In many centers, this type of complex reconstruction is avoided and a large ringed polytetrafluoroethylene (PTFE) graft is inserted.12
Arterial Repair—Upper Extremity
Injuries to the axillary, brachial, radial, or ulnar arteries account for approximately 45%-52% of peripheral arterial injuries treated in civilian trauma centers.8,9 Because of its length and exposed position in the upper extremity, injuries to the brachial artery are 3–3.5 times more common than those to the axillary artery.
With suspected or documented injuries to the axillary artery, it is helpful to cover the entire upper extremity in an orthopedic stockinette and place it at the side of the patient if an injury to the first or second portion is suspected. This will allow for more room for the operating team as well as relax the muscles around the shoulder girdle as dissection proceeds. With a suspected injury to the third portion of the axillary artery, the upper extremity is abducted at 90° and placed on an armboard.
The axillary artery starts at the lateral border of the first rib and becomes the brachial artery at the lateral border of the teres major muscle. The operative approach varies depending on whether the arterial injury is located in the first portion (lateral border of the first rib to medial border of the pectoralis minor muscle), second portion (behind the pectoralis minor muscle), or third portion (lateral border of the pectoralis minor muscle to lateral border of teres major muscle). Injuries to the first or second portions that are not actively hemorrhaging are most commonly approached through an infraclavicular incision centered on the midclavicle.46 After splitting the upper fibers of the pectoralis major muscle in a transverse fashion, the clavipectoral fascia is divided. Proximal arterial control is obtained by retracting the anteriorly positioned axillary vein in an inferior fashion and placing a vessel loop around the axillary artery just inferior to the clavicle. Distal control is obtained, as needed, by extending the infraclavicular incision into an incision in the deltopectoral groove. Should active hemorrhage from the second portion of the artery occur during dissection or the tamponaded injury is in the second portion, lateral retraction of the tendon of the pectoralis minor tendon is necessary. Persistent inadequate exposure of this arterial location mandates division of the tendon of the pectoralis minor muscle near the coracoid process to preserve the medial pectoral nerve. An injury in the third portion can be approached through the aforementioned incision in the deltopectoral groove. An alternate, but uncommonly utilized, approach involves a lateral pectoral incision along the edge of the pectoralis major muscle.
Injuries to the axillary artery, particularly those that are bleeding actively, are challenging because of the adjacent cords of the brachial plexus. The blind application of angled vascular clamps often entraps portions of the cords, leading to a partial brachial plexopathy for 12–24 months. Therefore, elevation of the injured artery proximally and distally with vessel loops is mandatory before vascular clamps are applied. The second problem in dealing with injuries at this location is the somewhat fragile nature of the axillary artery. This artery is rarely involved with substantial atherosclerosis and is extraordinarily soft with a consistency that sometimes approaches that of the subclavian artery. Lateral repairs performed with suture bites that are too thin or end-to-end repairs performed under tension will lead to sutures tearing through when vascular clamps are released.
The brachial artery starts at the lateral border of the teres major muscle, courses through the medial arm, and bifurcates at the radial tuberosity of the forearm. The operative approach in the arm has been described previously. When an injury occurs near the elbow, the standard S-shaped incision extending from the medial biceps–triceps groove is used. This incision crosses the antecubital fossa and then turns longitudinally beyond the midaspect of the volar side of the forearm. The fascia overlying the neurovascular bundle medially and the bicipital aponeurosis beneath the antecubital fossa are divided to allow for complete exposure of the brachial artery proximal to its bifurcation.
There is a logical sequence for performing a complex repair (end-to-end anastomosis or insertion of a graft) of the axillary or brachial artery after proximal and distal arterial control has been obtained with vessel loops (Fig. 31.3). When an end-to-end anastomosis is to be performed, a posterior knot is usually placed at 6 o’clock. If exposure is limited, as in an end-to-end anastomosis performed near the clavicle, it is helpful to perform the first one-third of the posterior anastomosis in an open fashion (no posterior knot) in both directions to allow for complete visualization of all suture bites.47 After this portion of the anastomosis is complete, the ends of the artery are pushed together as both sutures are pulled tight. Because both ends of the artery are now stabilized, a No. 5 or 6 Fogarty embolectomy catheter is passed proximally and distally to remove any thrombotic or embolic material. Approximately 10–15 mL of heparinized saline solution (50 U/mL of solution) is then injected into each end of the artery, and the vascular clamps are reapplied. The remaining two-thirds of the anastomosis is completed by running one end of the continuous suture along one side and the other end along the other side. The last few loops of suture, however, are left loose to allow for flushing before the anterior knot is tied. The proximal vascular clamp is removed for flushing and then reapplied. The distal vascular clamp is removed to allow for flushing as well. As air is evacuated by the distal flushing, the two suture ends are pulled up tight and tied. Once the first knot has been tied, the proximal arterial clamp is released. Bleeding from suture holes is controlled by the application of oxidized regenerated cellulose (Surgicel, Johnson & Johnson Medical, Inc., Arlington, TX) or Avitene (Med Chem Products, Inc., Woburn, MA). Because distal in situ thrombosis is very unusual during proximal arterial repairs in the upper extremity, the return of palpable pulses at the ipsilateral wrist obviates the need for a completion arteriogram. When distal pulsations are diminished or absent after removal of the vascular clamps, a completion arteriogram is mandatory.
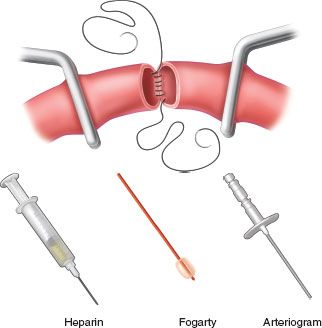
FIGURE 31.3. Fine points in peripheral arterial repair include use of small vascular clamps or Silastic vessel loops, open anastomosis technique, regional heparinization, passage of a Fogarty catheter proximally and distally, and arteriography on completion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








