Diseases of the Thyroid and Parathyroid Glands
Muriel N. Nathan PhD, MD
This chapter will discuss thyroid and parathyroid diseases. Because of the complexity of this information, the chapter will be divided into several parts. First, hyperthyroidism, including Graves’ disease, goiter, subacute thyroiditis, and thyroid storm, will be discussed. This will be followed by hypothyroidism, including chronic autoimmune thyroiditis, postpartum and treatment-related hypothyroid conditions, rarer forms of the disease, secondary hypothyroidism, and thyroid resistance syndromes. Thyroid cancer will be discussed. Hyperparathyroidism and the rarer state of hypoparathyroidism will follow. After a discussion of parathyroid diseases, community-based resources will be covered at the chapter’s end.
THE THYROID GLAND
Thyroid disease is a common cause of outpatient visits to primary care providers. The size and texture of the gland can change. Goiter is a generalized enlargement of the thyroid gland, whereas a thyroid nodule is a focal enlargement. Symptoms can be caused by overactivity of the thyroid gland (hyperthyroidism) or underactivity (hypothyroidism). Autoimmune diseases can affect the function of the thyroid gland and can lead to hyperthyroidism (Graves’ disease) or hypothyroidism (Hashimoto’s thyroiditis). Lack of iodide in the diet or excessive iodine loading can lead to alterations in thyroid hormone synthesis.
HYPERTHYROIDISM
Anatomy, Physiology, and Pathology
The adult thyroid gland is composed of two lobes on each side of the trachea and a connecting isthmus. Some people have a pyramidal lobe, a remnant of the thyroglossal duct, that extends superiorly from the isthmus to the hyoid (Pintar, 1996). The normal gland weighs about 20 g, with each lobe about 5 cm long and 3 cm wide. It is palpable in most persons.
Connective tissue divides the thyroid into irregular portions. Thyroid hormone is synthesized by the follicular cells and stored in the colloid, where it can be taken up by the thyroid follicular cells for recycling or released into nearby capillaries. Thyroid tissue also contains vascular and nervous tissue elements and parafollicular cells that secrete calcitonin (Pintar, 1996).
The thyroid gland is diffusely enlarged in Graves’ hyperthyroidism. Microscopic evaluation shows follicular hyperplasia, the colloid content is reduced, and the cells look activated, with more numerous mitochondria and increased microvilli at the cell surface (Pintar, 1996). In subacute thyroiditis, biopsy of the gland shows thyroid tissue infiltrated by granulocytes, monocytes, and giant cells. In some cases, lymphoid germinal centers are found (Lazarus, 1996).
Thyroid hormones are formed from two tyrosine molecules that are iodinated. Tetraiodothyronine or thyroxine (T4) is produced only by the thyroid gland, whereas triiodothyronine (T3) is produced by the thyroid and by conversion of T4 at extrathyroidal sites. Thyroid hormone synthesis is dependent on the dietary intake of iodide and its subsequent transport to the follicular cells. To remain euthyroid, the minimal recommended intake of iodide is 150 mcg/day (Reed & Pangaro, 1995). In the United States, iodide is added to salt and flour, and dietary intake is usually 500 to 800 mcg/day (Reed & Pangaro, 1995). Daily production of T4 is about 100 mcg/day, half of which is then converted to T3 (Reed & Pangaro, 1995).
T3 and T4 travel in the bloodstream bound to serum proteins, particularly thyroxine-binding globulin, which binds T4 more avidly than T3. At the level of the cell, T4 and T3 dissociate from the binding proteins and enter as free hormones. Membranes within the cells contain receptors for T4 and T3, and at the nucleus receptors have a 10-fold increased avidity for T3; there, the hormone stimulates mRNA production. Thyroid hormone ultimately stimulates thermogenesis by increased ATP use and promotes the synthesis of many structural proteins (Usala, 1995).
Thyroid hormone synthesis is regulated by the availability of intrathyroidal iodide and by thyrotropin or thyroid-stimulating hormone (TSH) (Scanlon & Toft, 1995). TSH is secreted by pituitary thyrotrophs. Its alpha subunit is identical to luteinizing hormone, follicle-stimulating hormone, and human chorionic gonadotropin, but its beta subunit is unique. As in all hormone systems, its secretion is pulsatile and its release is stimulated by thyrotropin-releasing hormone (TRH), which is made in the paraventricular nucleus of the hypothalamus. Low levels of free T4 and free T3 stimulate TRH and TSH release by negative feedback. Dopamine and somatostatin, on the other hand, inhibit TSH secretion (Scanlon & Toft, 1995).
Epidemiology
HYPERTHYROIDISM
Hyperthyroidism is a common disorder, occurring in about 19 per 1000 women and 1.6 per 1000 men in North America (Burman, 1995). The annual incidence rate is 3 per 1000 women (Burman, 1995). In Europe, the annual incidence rate
is 25 per 100,000 people (Hay & Morris, 1996). Graves’ disease is the most common cause of hyperthyroidism. It most commonly occurs in young women in the reproductive years, but it can occur in children and in men and women of any age. In the older patient (>40 years), hyperthyroidism is usually caused by a toxic multinodular goiter. The prevalence of toxic multinodular goiter appears to vary depending on the rate of endemic goiter. In areas of iodine deficiency (eg, Malmo, Sweden), toxic multinodular goiter is more common, occurring in 9% to 21% of the patients with thyrotoxicosis. In areas of iodine excess (eg, Great Britain), the rate of toxic nodular goiter is 3% (Hay & Morris, 1996).
is 25 per 100,000 people (Hay & Morris, 1996). Graves’ disease is the most common cause of hyperthyroidism. It most commonly occurs in young women in the reproductive years, but it can occur in children and in men and women of any age. In the older patient (>40 years), hyperthyroidism is usually caused by a toxic multinodular goiter. The prevalence of toxic multinodular goiter appears to vary depending on the rate of endemic goiter. In areas of iodine deficiency (eg, Malmo, Sweden), toxic multinodular goiter is more common, occurring in 9% to 21% of the patients with thyrotoxicosis. In areas of iodine excess (eg, Great Britain), the rate of toxic nodular goiter is 3% (Hay & Morris, 1996).
Excessive exogenous thyroid ingestion is a common cause of mild hyperthyroidism (Burman, 1995; Nuovo, 1995). This ingestion could be prescribed or surreptitious, and can be found in many persons taking doses of 0.2 mcg or greater of L-thyroxine, 0.075 mg/day or more of L-triiodothyronine, or more than 180 mg/day (3 grains) of thyroid extract.
CLINICAL PEARL
Thyrotoxicosis is more likely to develop in persons with autonomous nodules or persons taking thyroid extract or combinations of T3 and T4, because the T4 measurements in the serum underestimate the dose of thyroid hormone delivered (Burman, 1995; Hay & Morris, 1996). Although this hyperthyroidism is usually caused by ingestion of thyroid medication, there have been epidemics of hyperthyroidism caused by the ingestion of meat from neck strap muscles contaminated with thyroid gland (Burman, 1995).
Hyperthyroidism can also be caused by exposure to iodine in persons with a degree of thyroid autonomy (toxic nodules, multinodular goiter) (Burman, 1995; Hay & Morris, 1996). The hyperthyroidism can occur 3 to 8 weeks after an iodine load (eg, use of expectorant or intravenous contrast) and persists for several months. Likewise, use of amiodarone for cardiac arrhythmias can induce thyroiditis and cause hyperthyroidism, which is quite difficult to treat (Bartelena, 1996), because the iodine content of the drug is high (33%) and the half-life is prolonged (55 days). Radioactive iodine cannot be used in these cases, but antithyroid medication and steroids are helpful until the drug is eliminated and hyperthyroidism remits (Bartelena, 1996).
Rare cases of hyperthyroidism can be caused by excessive TSH secretion from a pituitary adenoma (Burman, 1995; Franklyn, 1994). These patients have goiter but no ophthalmopathy. Tumors of trophoblastic tissue (hydatidiform mole, choriocarcinoma) can also cause hyperthyroidism, because the human chorionic gonadotropin produced by such tumors has a weak thyroid-stimulating ability (Burman, 1995). Ectopic thyroid tissue within the ovary (struma ovarii, a dermoid tumor or teratoma of the ovary) can cause hyperthyroidism (Burman, 1995). Here, radioisotope scanning will show no uptake in the neck, but rather uptake in the pelvic area; this is diagnostic. In rare cases of thyroid cancer, patients will become hyperthyroid if they have a large amount of metastatic deposits of thyroid tissue (Burman, 1995).
GRAVES’ DISEASE: PATHOPHYSIOLOGY
Graves’ hyperthyroidism is an autoimmune disease caused by the production of antibodies that bind to the thyroid cell’s TSH receptor, altering its function. It is believed that an intrinsic thyroidal cell defect changes immune cell function, activating a normally suppressed population of B lymphocytes (Burman, 1995). The B lymphocytes produce the autoantibody thyrotropin-binding inhibitory immunoglobulin, which mimics the action of TSH (Burman, 1995). This causes excessive production of thyroid hormone and growth of the thyroid gland. Many believe that autoimmune thyroid disease, Graves’ and Hashimoto’s thyroiditis (discussed under hypothyroidism), although not immunogenetically identical, represent opposite ends of a single illness. Both diseases have strong genetic components, involve lymphocytic infiltration of the gland, and elaborate autoantibodies (Burman, 1995).
Graves’ hyperthyroidism appears to be an inherited disease because there is an increased frequency of certain genetic markers (histocompatibility complexes) in affected persons, especially HLA-B8 and DR3. In addition, there is a high concordance rate for the disease among monozygotic twins (Burman, 1995). Although autoantibodies cause the goiter and hyperthyroidism of Graves’ disease in a genetically predisposed person, not all people with these genetic markers develop autoimmune thyroid disease. The development of disease may be linked to an environmental stimulus that permits expression of the gene or allows the thyroidal defect. At present this stimulus is unidentified, but it may involve viral infections, sex hormone levels, or stress, factors that could change immune cell function.
History and Physical Examination
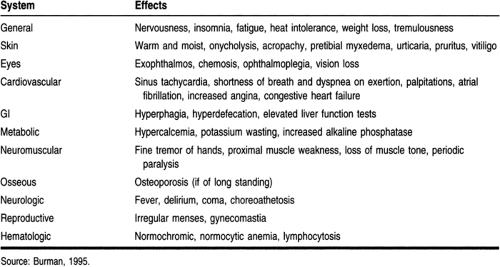
Hyperthyroidism or thyrotoxicosis is the clinical syndrome that results when tissues are exposed to excessive thyroid hormone. Hyperthyroidism can have multiple causes and can vary in severity and length of illness. Its causes are presented in Table 19-1.
Symptoms are caused by the effects of excessive thyroid hormone on the various organ systems. Although different causes of hyperthyroidism may produce similar symptoms and signs of disease, it is important to differentiate the cause of the hyperthyroidism to devise the best treatment strategy.
Patients with hyperthyroidism display restlessness, anxiety, and emotional lability (Burman, 1995; Singer et al, 1995). Their ability to concentrate is diminished, as is their exercise tolerance because of proximal muscle weakness. They may notice a hand tremor, excessive perspiration, heat intolerance, and weight loss despite an increase in appetite. About 20% of patients gain weight. Increased gut motility leads to an increased frequency of bowel movements, but not true diarrhea.
CLINICAL WARNING
Hyperthyroidism can occasionally cause potassium wasting and loss of muscle tone, which results in episodes of localized or generalized weakness called periodic paralysis (Burman, 1995). Periodic paralysis is most often seen in patients who are Asian, and episodes of paralysis can be brought on by strenuous activity, consumption of carbohydrates or alcohol, or use of medications such as insulin.
Patients usually seek care because of the thyroid enlargement, eye changes, cardiopulmonary symptoms, or difficulty at work or in their personal relationships. Table 19-2 presents additional symptoms and signs of hyperthyroidism.
Graves’ disease has some unique signs and symptoms. The disease is characterized by a triad of findings: hyperthyroidism, diffuse thyroid enlargement (goiter), and infiltrative ophthalmopathy and dermopathy (pretibial myxedema) (Burman, 1995). Infiltrative ophthalmopathy is present in up to 50% of patients with Graves’ disease, and it can develop before hyperthyroidism is evident (Burman, 1995; Prummel & Wiersinga, 1995). Ophthalmopathy is caused by the accumulation of glycosaminoglycans in the orbital fat, connective tissue, and muscle (Bahn & Heufelder, 1993). There is infiltration of the tissues by lymphocytes and plasma cells and edema (Bahn & Heufelder, 1993). Symptoms include:
A sensation of grittiness in the eyes
Blurred vision
Photophobia
Diplopia
Increased lacrimation
A feeling of increased orbital pressure (Burman, 1995; Bahn & Heufelder, 1993).
Signs of ophthalmopathy are:
Bilateral proptosis, usually symmetrical
Periorbital and conjunctival edema
Limited ocular movement, especially upward gaze
CLINICAL PEARL
It is believed that both uncontrolled thyrotoxicosis and hypothyroidism worsen ophthalmopathy (Torring, 1996; Tallstedt, 1992).
Infiltrative dermopathy is a rare manifestation of Graves’ disease, found in less than 10% (Burman, 1995). These nontender lesions are scaly plaques that are erythematous or hyperpigmented on the feet, ankles, or tibial region. Biopsy reveals epidermal atrophy, fibrosis, and mucinous edema of the dermis (Burman, 1995). Even rarer is thyroid acropachy, the clubbing of fingers and toes from subcutaneous fibrosis and periosteal bone formation involving the phalanges, metatarsals, and metacarpals (Burman, 1995).
On physical examination, the skin of a hyperthyroid patient is warm and smooth. Lid lag and lid retraction occur and can be found in hyperthryoidism of any cause (Burman, 1995). Infiltrative ophthalmopathy with protrusion of the eye and limited movement of the globe is most often caused by Graves’ hyperthyroidism or autoimmune thyroid disease. Thyroid enlargement is generally found but can be absent in 20% of patients with Graves’ (Burman, 1995). Because of increased blood flow to the hyperactive gland, a bruit or venous hum can be auscultated over the gland. Systolic hypertension, sinus
tachycardia, and atrial fibrillation can be found. There is a fine hand tremor that is apparent when the hands are stretched out in front of the examiner (Burman, 1995; Singer et al, 1995).
tachycardia, and atrial fibrillation can be found. There is a fine hand tremor that is apparent when the hands are stretched out in front of the examiner (Burman, 1995; Singer et al, 1995).
TOXIC MULTINODULAR GOITER
Hyperthyroidism is also commonly the later stage of a multinodular goiter (Plummer’s disease) (Hay & Morris, 1996; Singer et al, 1995; Franklyn, 1994). In toxic multinodular goiter, the gland is more irregular and nodular than in Graves’ disease. Patients thus present with complaints of an enlarging neck mass or difficulty swallowing. Hyperthyroidism usually presents in an insidious manner and there is no infiltrative ophthalmopathy or dermopathy (Hay & Morris, 1996; Singer et al, 1995). In some cases, hyperthyroidism can follow exposure to drugs rich in iodine, such as intravenous contrast used in a computed tomography (CT) examination, the use of cough syrup with expectorants, or the use of amiodarone, an antiarrhythmic drug.
A few patients present with hyperthyroidism from a toxic (autonomous) nodule. This is found mostly in patients with a thyroid nodule that measures 3 cm or greater who are younger than 20 or older than 60 years (Hay & Morris, 1996).
SUBACUTE THYROIDITIS
Subacute thyroiditis (nonsuppurative thyroiditis, granulomatous thyroiditis, or De Quervain’s disease) is an inflammatory disease of the thyroid gland that can cause overt hyperthyroidism. It is the most common cause of pain and tenderness of the thyroid gland (Lazarus, 1996). Its onset can be abrupt or gradual, and the pain is over the thyroid gland, with radiation to the jaw, throat, and ears. Many patients give a history of a recent upper respiratory infection, and they may give a history of fever, sore throat, and myalgias. The pain and hyperthyroidism are transient, subsiding in weeks to 2 to 3 months. Transient hypothyroidism may follow this period of hyperthyroidism because of leakage of stored hormone from the gland and a disruption of the biosynthesis of hormone. Permanent hypothyroidism is unusual (Lazarus, 1996).
Thyroid inflammatory disease can present in a silent or painless mode (Lazarus, 1996). This usually occurs in the postpartum period, and it can be difficult to differentiate painless thyroiditis from early Graves’ disease. Nonetheless, postpartum thyroiditis is transient and does not lead to infiltrative ophthalmopathy. Women will report faster-than-expected weight loss after parturition, mood changes, anxiety, and palpitations that may all be erroneously attributed to the postpartum blues.
Diagnostic Studies
LABORATORY TESTING
Assays for Total T4 and T3
The concentrations of total T4 and T3 are measured in the blood using radioimmunoassay. Serum total T4 and T3 reflect hormonal production and also the serum concentration of thyroid hormone-binding proteins. When the level of serum thyroxine-binding globulin (thyroid hormone-binding protein) is increased, serum total T4 and T3 are increased, whereas the free hormones are not.
Estimation of Serum Free T4and T3: Free Thyroxine Index
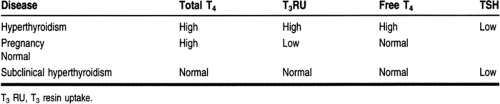
The amount of free T4 can be estimated by separation of the free from the bound T4 by a semipermeable membrane in equilibrium dialysis, a very expensive procedure, or by partition of tracer T3 between serum proteins and a nonspecific solid-phase matrix (resin uptake). A serum free T4 index can be calculated from the serum total T4 and the thyroid hormone-binding ratio. Labeled T3 is used because it is less tightly bound to proteins. The thyroid hormone-binding ratio or T3 resin uptake estimates the number of unoccupied serum protein binding sites. Table 19-3 presents these values in hyperthyroidism and other states.
In hyperthyroidism, the TSH level is low or undetectable when using the ultrasensitive assays that are now available. The finding of a low TSH with elevated free thyroid hormones confirms the state of hyperthyroidism (Burman, 1995; Hay & Morris, 1996; Singer et al, 1995; Franklyn, 1994).
CLINICAL WARNING
Finding an elevated total T4 may not indicate hyperthyroidism, because pregnancy or the use of conjugated estrogens increases the level of thyroxine-binding globulin (Burman, 1995). It is important in women with elevated total T4 levels that hyperthyroidism is confirmed by the finding of a low TSH value.
There are some patients with a low TSH level who have high T3 but not T4 levels. T3 thyrotoxicosis is more likely to occur in patients with a toxic thyroid nodule, but it can be seen in hyperthyroidism of any cause. Other patients have isolated elevations of T4 and a low TSH level. In these cases, there is an intervening serious illness that inhibits T4 to T3 conversion (euthyroid sick syndrome) or the concomitant use of a drug, such as propranolol, that inhibits T4 to T3 conversion, so that T3 levels are normal. Some persons show subclinical hyperthyroidism, in which TSH levels are low but free hormone levels are normal. This is often found in patients with autonomous thyroid function, such as euthyroid Graves’, thyroid adenoma, or multinodular goiter (Burman, 1995; Hay & Morris, 1996; Singer et al, 1995).
Some patients on routine testing have low TSH values without clinically apparent disease. A low TSH level can be normal for that population, but free T4 and total T3 levels should be checked to look for thyrotoxicosis. Asymptomatic patients may
have apathetic hyperthyroidism, which is seen more frequently in the elderly, or thyroid autonomy (toxic nodule or toxic multinodular goiter) or sick euthyroidism.
have apathetic hyperthyroidism, which is seen more frequently in the elderly, or thyroid autonomy (toxic nodule or toxic multinodular goiter) or sick euthyroidism.
TSH values are also low in secondary hypothyroidism, but the T4 (free hormone) level is not elevated (see section on hypothyroidism). A normal or elevated TSH value in the presence of elevated free thyroid hormone levels indicates excessive pituitary secretion of TSH from a pituitary adenoma or thyroid resistance syndrome (Singer et al, 1995).
Thyroid antibodies are also found in the blood of patients with Graves’ hyperthyroidism. The antibodies that are most specific are those that bind to TSH receptors, which include thyrotropin-binding inhibitor immunoglobulins or thyroid-stimulating immunoglobulins. Thyroid antibodies may be present during periods of remission, and their absence does not seem to be predictive for permanent remission (McIver, 1996). Antithyroid antibodies that bind to other cellular components are called antithyroglobulin and antimicrosomal, or thyroid peroxidase antibodies. These levels are also elevated, although titers are usually lower than those in patients with autoimmune thyroiditis (Burman, 1995) (see section on hypothyroidism).
Other laboratory abnormalities found in patients with hyperthyroidism include increased liver function tests, specifically aminotransferase and alkaline phosphatase; high calcium levels; glucose intolerance (patients with diabetes may note that their insulin requirements increase); and anemia with a relative decrease in granulocytes and an increase in lymphocytes. Patients with subacute thyroiditis have an elevated erythrocyte sedimentation rate (Lazarus, 1996; Burman, 1995; Franklyn, 1994). Thyroglobulin levels are increased, unless the hyperthyroidism is caused by exogenous intake of thyroid hormone.
RADIONUCLIDE IMAGING STUDIES
Most patients with hyperthyroidism have increased iodine uptake 24 hours after a tracer dose of radioactive iodine (131I). The exceptions, showing low uptake during clinical hyperthyroidism, are patients with thyroiditis or patients who have taken thyroid hormone or received iodine loads (intravenous contrast or amiodarone) before the test. Uptakes are done to confirm the presence of hyperthyroidism and are not necessary for the diagnosis of classic cases of Graves’ disease (Lazarus, 1996; Burman, 1995; Singer et al, 1995). Uptakes are also done to differentiate cases of thyroiditis from Graves’ if clinical cues are inadequate to distinguish these causes of hyperthyroidism.
Thyroid scans that allow images of the gland to be generated are done with labelled iodine (123I) or pertechnetate (technetium, 99Tc). Both radioisotopes emit gamma particles that are detected by a gamma camera. 99Tc is less expensive and has a shorter half-life, allowing less radiation exposure. In Graves’ disease, the distribution of radionuclide within the thyroid is homogeneous, whereas toxic adenomas or toxic multinodular goiters are heterogeneous, showing some areas of increased uptake and other areas of decreased uptake (Burman, 1995; Hay & Morris, 1996). It is important that patients with areas of decreased uptake on thyroid scanning (cold nodules) are evaluated for thyroid cancer. Although most have multinodular goiters, some patients will harbor thyroid carcinoma in nonfunctioning tissue. Patients with hypothyroidism or thyroiditis demonstrate low or no uptake of radionuclide, and thyroid scans either cannot be generated or may look patchy (Lazarus, 1996).
COMPUTED TOMOGRAPHY AND MAGNETIC RESONANCE IMAGING
Graves’ disease causes thickening of the eye muscles in more than 50% of patients; this can be detected by CT or magnetic resonance imaging (MRI) of the orbits. Orbital CT or MRI is particularly useful when the patient has euthyroid Graves’ (normal thyroid function) or unilateral eye involvement, where the etiology of the proptosis may not be easily recognized. Although CT scanning does expose the lens to radiation, it is the preferred imaging modality because of its lower cost and its ability to provide bony anatomic detail.
Treatment Options, Expected Outcomes, and Comprehensive Management
Graves’ disease is characterized by exacerbations, perhaps provoked by physical or emotional stress, and spontaneous remissions, where long periods of euthyroidism (normal thyroid function) can occur after the withdrawal of medical therapy. Remissions occur in about 25% of patients and are more likely with:
Hyperthyroidism of recent onset
Mild disease
Modest thyroid enlargement
Treatment strategies depend on the expected length of disease and its severity.
CLINICAL PEARL
Graves’ disease has no one specific therapy, and it is difficult to predict who will go into remission and how long a remission will last. Thus, most patients are offered either antithyroid drug therapy or ablative treatment with radioactive iodine. The latter option is used most often in older patients with Graves’. Antithyroid drugs bring about euthyroidism more rapidly, do not cause permanent thyroid damage, and are inexpensive. Thus, antithyroid drugs are usually the initial treatment plan. If a side effect to medication occurs, or if hyperthyroidism is not controlled within a reasonable length of time, drug therapy can be withdrawn and radioactive iodine given. Radioactive iodine almost always results in hypothyroidism, which can occur any time after treatment (Burman, 1995; Franklyn, 1994; Torring, 1996).
CLINICAL WARNING
Radioactive iodine is contraindicated in pregnant women, and pregnancy should be deferred for at least 3 months after treatment.
DRUG THERAPY FOR HYPERTHYROIDISM
Antithyroid drugs that are available in the United States include propylthiouracil (PTU) and methimazole (Tapazole, MMI). Both work by inhibiting thyroid hormone synthesis, blocking iodine oxidation and iodotyrosine coupling. Both may inhibit antithyroid antibody production by inhibiting lymphocyte function. PTU also blocks, in a limited fashion, T4 to T3 conversion (Burman, 1995; Franklyn, 1994). Both drugs are absorbed
from the gut rapidly, but MMI is better concentrated in the gland and metabolized slowly (Singer et al, 1995). MMI is usually given as 10 to 40 mg/day in two or three doses; per day PTU is given in two or three doses a day at 300 to 1200 mg. The larger the goiter, the larger the dose given.
from the gut rapidly, but MMI is better concentrated in the gland and metabolized slowly (Singer et al, 1995). MMI is usually given as 10 to 40 mg/day in two or three doses; per day PTU is given in two or three doses a day at 300 to 1200 mg. The larger the goiter, the larger the dose given.
There is biochemical improvement in 2 to 6 weeks and clinical improvement in 4 to 6 weeks (Burman, 1995; Franklyn, 1994; Torring, 1996). Most patients reach euthyroidism in 8 to 10 weeks, and the dose can be reduced by 25% to 50% (Burman, 1995). Initial therapy is to inhibit thyroid synthesis altogether until thyroid stores are depleted. Once euthyroidism is reached, the goal is partial inhibition, being careful to prevent hypothyroidism, which could aggravate thyroid enlargement or orbitopathy. Failure to control hyperthyroidism is often caused by noncompliance with therapy or inadequate doses of drug; often T4 levels fall, but T3 levels are still elevated, leading to persistent hyperthyroidism.
Patients are seen every 4 to 6 weeks until euthyroid on the medication and then every 3 months thereafter (Burman, 1995; Franklyn, 1994). Antithyroid medication is given for up to 24 months or longer to ensure remission; shorter periods of treatment usually lead to a recurrence of the hyperthyroidism within months of drug withdrawal. Franklyn (1994) states that one study that looked at patients 1 year after stopping treatment for Graves’ disease found that 31% of patients who used antithyroid medication for 6 months were in remission, but 82% of patients on medication for 2 years were still euthyroid.
CLINICAL PEARL
There is no test that reliably predicts remission, although normal free hormone levels on low doses of medication and a dramatic decrease in the goiter size are good indicators (Franklyn, 1994).
An alternative treatment protocol for Graves’ disease is to give high doses of antithyroid medication; when the patient responds, instead of decreasing the dose, thyroid supplement is added to prevent hypothyroidism. This dual therapy of “blockade and add back” allows higher doses of antithyroid drug to be given to suppress antithyroid antibody formation, supposedly increasing the rate of remission without causing hypothyroidism. The efficacy of this therapy has been shown in Japan and Europe. In a European study, medical therapy using antithyroid drug alone for 6 months and then with thyroxine for an additional 12 months was successful during a 4-year follow-up in 58% of young adults and 66% of adults over age 35 (Torring, 1996). This same therapy has not been as successful in North America, where the recurrence rate of hyperthyroidism (about 30%) was similar in patients given antithyroid medication and those given antithyroid medication followed by antithyroid medication plus thyroxine (McIver et al, 1996).
CLINICAL WARNING
All patients taking antithyroid medication should be warned to look for the minor and major side effects of the medication. A common minor reaction, especially to PTU, is an itchy skin rash. Often the rash improves with antihistamine use and as the hyperthyroidism is controlled, but bothersome skin eruptions may mandate changing to MMI or stopping medication and using radioablation to reverse hyperthyroidism. More serious side effects, such as hepatitis, fever, and agranulocytosis (which occurs in 0.3%), require immediate cessation of medication and often hospitalization until blood tests improve. White blood counts should be obtained as a baseline because Graves’ disease can produce mild leukopenia (Burman, 1995; Singer et al, 1995; Franklyn, 1994).
Inorganic iodine can decrease thyroid hormone levels by inhibiting the release of T4 and T3 by the thyroid gland (Burman, 1995; Franklyn, 1994). Iodide also blocks T4 to T3 conversion. This usually requires 5 to 10 mg of iodide daily. Escape from the antithyroid effects of iodide occurs within 7 to 14 days, but it is useful in patients with severe hyperthyroidism. Iodide is given several hours after starting antithyroid medication to avoid stimulating thyroid hormone synthesis. A saturation solution of potassium iodide (SSKI, 50 mg iodide/drop) or oral iodinated-contrast agents (Telepaque, 500 to 1000 mg b.i.d.) can be used (Burman, 1995).
Beta-adrenergic antagonists reverse many of the clinical symptoms and signs of hyperthyroidism. Propranolol is often used in a dosage of 40 to 160 mg/day to treat palpitations, tachycardia, nervousness, and hand tremors. Propranolol used alone does not reverse the catabolic effects of hyperthyroidism. It is the treatment of choice in thyroiditis and to control the symptoms of hyperthyroidism before 131I therapy becomes effective (Lazarus, 1996; Burman, 1995; Singer et al, 1995; Franklyn, 1994).
RADIOIODINE ABLATION
Radioactive iodine (131I) is an effective treatment for Graves’ disease because it reduces the volume of functioning thyroid tissue. The advantages of using radioactive ablation over antithyroid medication are its lack of side effects and its efficacy. Nonetheless, hyperthyroidism may not be reversed for many months. Thus, severely ill patients may need to be pretreated with antithyroid medication until they are more stable (Burman, 1995; Franklyn, 1994; Torring, 1996). Before therapy, iodine-containing drugs and contrast agents must be avoided. The usual dose of 131I is 8 to 12 mCi for Graves’ disease, delivering 10,000 to 20,000 rad to the thyroid (Burman, 1995). Higher doses of 131I (12 to 15 mCi) are given to patients who used antithyroid drugs before radioablation (Burch, 1994). Acute exacerbation of hyperthyroidism may occur within the first 2 weeks after treatment from radiation-induced thyroiditis (Franklyn, 1994). There may be some swelling of the goiter and neck pain, which responds to aspirin or steroids. Persistent hyperthyroidism may occur, especially if lower doses are used for treatment. Clinical and biochemical improvement occurs within 2 to 3 months; most patients are euthyroid or hypothyroid within 3 to 6 months. Hypothyroidism ensues in about 80% of treated persons within the following year, and 2% per year thereafter. Thyroidal underactivity is caused by radiation necrosis and the failure of the surviving cells to replicate.
CLINICAL WARNING
The primary care provider should refer any patient needing radioiodine ablation to a thyroid specialty practice for evaluation and treatment.
Concerns that 131I can cause secondary thyroid cancers or other tumors has not been borne out in the literature (Burman, 1995; Franklyn, 1994). There may be a risk for gastric carcinoma in patients treated with 131I for Graves’ who have a family history of gastric cancer and pernicious anemia. Nonetheless, because of the concerns about potential gonadal radiation and leukemia, 131I is not the preferred treatment for children and adolescents or women in their reproductive years. Adult men may not face the same risk after exposure to 131I because the testicles are farther removed from the bladder than the ovaries and because the testicles manufacture new spermatocytes every 90 days.
CLINICAL WARNING
Pregnant women are not candidates for radioablation because the radioactive iodine can cross the placental barrier and destroy the fetal thyroid. Women who are fertile need to have a pregnancy test before treatment with 131I.
SURGICAL ABLATION
Surgical treatment for hyperthyroidism is indicated for pregnant women who cannot tolerate antithyroid medication (Burman, 1995); patients with large goiters, especially those with compressive symptoms (Hay & Morris, 1996); patients intolerant of antithyroid medication who do not want radioactive iodine (Torring, 1996); and patients found to have coexistent suspicious nodules (Burman, 1995). Surgery can be preceded by short-term antithyroid medication, or in the nonpregnant patient iodide. Surgery is more expensive than treatment with radioiodine. Postoperative complications include vocal cord paralysis and transient or permanent hypocalcemia from hypoparathyroidism. Subtotal thyroidectomies, although safer in terms of less risk of vocal cord paralysis and hypoparathyroidism, may lead to recurrent hyperthyroidism because the thyroid remnant can be stimulated by remaining antithyroid antibodies. Many surgeons thus prefer to do near-total thyroidectomies (Burman, 1995) because it is difficult to reoperate in the neck. The recurrence rate for hyperthyroidism is about 3% to 8%; it tends to occur more than 5 years after surgery (Torring, 1996).
CLINICAL WARNING
All patients who undergo near-total thyroidectomies will be on lifelong thyroid hormone replacement and need to be followed for recurrence and adjustment of replacement by their primary care provider.
Surgical decompression is indicated if ophthalmopathy threatens vision.
CLINICAL PEARL
Periorbital edema from Graves’ ophthalmopathy can be improved by using mild diuretics at night and sleeping with the head raised. Methylcellulose (0.5%) drops can also be used for eye irritation (Burman, 1995; Bahn & Heufelder, 1993). Ophthalmopathy that is more severe is treated with oral steroids or orbital irradiation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree