Myeloproliferative Diseases
Elaine B. Owen R.N.C.S., M.S.N., A.O.C.N.
Myeloproliferative disorders are characterized by a malfunction of the bone marrow. Both preleukemic dysfunction and acute leukemia can present with symptoms found in a primary care setting. The increased incidence, because of an aging population, and the chronic nature of these diseases mean that many more primary care providers are likely to have patients in their practices with bone marrow disorders.
Chronic leukemia and acute leukemia are malignant disorders, whereas nonmalignant disorders, characterized by various cytopenias and dysplastic cell features, include diseases such as myelodysplastic syndrome and myelofibrosis. Some consider myelodysplastic syndrome a malignant condition because long-term survival data are sparse. All of these disorders involve the hematopoietic system and lead to disruption of normal hematopoiesis. They can be characterized by autonomous proliferation of poorly differentiated cells or, in the case of the chronic leukemias, by uncontrolled expansion of mature cells. The more common problems associated with these diseases will be discussed.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Anatomy and Physiology
Hematopoiesis is the process of making blood cells. All the cellular components of blood come from a single cell line progenitor called stem cells. Stem cells reside in the bone marrow, but they also circulate in peripheral blood. They can be harvested from the peripheral blood, obviating the need to extract bone marrow for use in transplant situations. The potential for plurality of these stem cells has led them to be labeled pluripotent progenitor cells.
Stem cells have the ability to self-replicate or differentiate into one of several cell-specific progenitors. Self-replication ensures replenishment of the cell line. Differentiation of stem cells commits cells to a path of distinct function. A pluripotent stem cell can be committed to become, for instance, either a leukocyte or an erythrocyte. Thus, hematopoiesis is the process of proliferation and differentiation of these self-renewing, pluripotent progenitor cells.
Figure 39-1 illustrates the hematopoietic cascade (Shoemaker, 1993). When a stem cell divides, the daughter cells may in turn self-replicate or further differentiate. This succession of stem cells (progenitors) and their daughter cells is called a lineage.
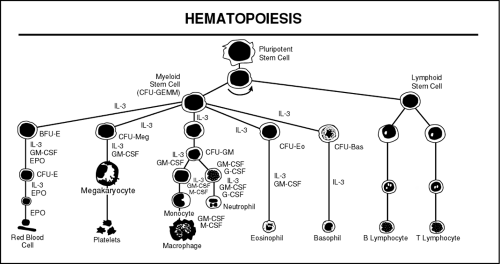 FIGURE 39-1 Hematopoietic Cascade. CFU-GEMM = colony-forming unit, granulocyte-erythrocyte-monocyte-megacaryocyte; IL-3 = interleukin-3; BFU-E = burst-forming unity, erythroid; CFU-Meg = CFU, megacaryocyte; CFU-GM = CFU, granulocyte-macrophage; CFU-Ec = CFU, eosinophil; CFU-Bas = CFU, basophil; GM-CSF = granulocyte-macrophage colony-stimulating factor; EPO = erythroprotein; M-CSF = macrophage CEF; G-CSF = granulocyte CSF. (Shoemaker, 1993) ( McCall, R.E. & Tankersley, C.M. (1998). Phlebotomy essentials, 2/e. Philadelphia: Lippincott-Raven Publishers ) |
Not all the factors that cause a stem cell to differentiate are known. It is known that as each tributary of the pathway branches or differentiates, there is an increasingly restrictive capacity to renew itself. The first major branching of the pathway divides into myeloid and lymphoid precursor cells. The myeloid cells are called colony-forming unit/granulocyte-erythrocyte-monocyte-megakaryocyte (CFU-GEMM); the lymphoid precursors are called simply lymphoid. The committed cells cannot be distinguished at this stage of hematopoiesis. Subsequent divisions and differentiations are necessary for these precursor cells to be recognizable.
The first recognizable cells in the CFU-GEMM line are myeloblasts (precursors of granulocytes and monocytes) and erythroblasts (precursors of red blood cells). Normally immature blast cells are not present in peripheral blood. A relatively small number of these immature cells are contained in the bone marrow and constitute less than 5% of the bone marrow space.
The cycle of blood cell reproduction, growth, maintenance, and destruction is a highly efficient and orderly process, tightly controlled by growth factors and suppressors. Abnormal proliferation and defective cell production of any cell line disrupt the delicate blood cell balance necessary to sustain the body’s good state of health.
Pathology
The adult leukemias constitute about 10% of all cancers. They are a heterogeneous group of diseases, with myeloid and lymphoid subclasses, and are broadly divided into acute and chronic disorders. The acute leukemias are characterized by autonomous proliferation of undifferentiated cells, whereas the chronic leukemias are distinguished by uncontrolled expansion of mature cells. Cellular proliferation genes and tumor suppressor genes are responsible for regulation of cellular growth. There are genes that also regulate apoptosis or programmed cell death. Bone marrow dysfunction may result from aberrant regulation of this homeostasis.
CHRONIC LEUKEMIA AND RELATED DISORDERS
Two types of chronic conditions affect the myeloid cell lineage, which consists of granulocytes, red cells, and platelets. Myeloproliferative disorders, the first type, are characterized by an efficient abnormal proliferation, resulting in an overproduction of mature cells. They include chronic myelocytic leukemia, polycythemia vera, essential thrombocythemia, and chronic myelofibrosis. Myelodysplastic syndromes (MDS), the second type, involve inefficient abnormal proliferation, resulting in an underproduction of mature, normal blood cells. MDS patients have a cellular marrow but low blood counts.
Myelofibrosis
Myelofibrosis, or fibrosis of the bone marrow, can be seen with malignant conditions such as the leukemias, lymphomas, and solid tumors, as well as nonmalignant conditions such as Paget’s disease, osteoporosis, lupus erythematosus, and chemical exposures.
Agnogenic myeloid metaplasia is another term used to describe myelofibrosis. Myelofibrosis is considered a neoplastic disorder because of chromosomal and genetic abnormalities, the presence of certain enzyme and cell defects, and its chronic progressive nature.
Agnogenic myeloid metaplasia is another term used to describe myelofibrosis. Myelofibrosis is considered a neoplastic disorder because of chromosomal and genetic abnormalities, the presence of certain enzyme and cell defects, and its chronic progressive nature.
In most presentations of myelofibrosis, an underlying disease state is the causative agent. Myelofibrosis also may occur de novo, in which extramedullary hematopoiesis is a prominent feature (Athens, 1993). Extramedullary hematopoiesis, or blood formation outside the marrow space, most commonly occurs in the spleen, although the liver, lymph nodes, skin, thymus, or other organs can be affected.
Chronic Myelogenous Leukemia
Chronic myelogenous leukemia (CML) is characterized by a proliferation of granulocytes, especially neutrophils, and the hallmark Philadelphia chromosome. This chromosome is the G group chromosome 22 missing a portion of the long arm, which has been translocated to the long arm of chromosome 9. The translocation activates a gene that produces a protein. This protein stimulates growth factor receptors, inducing uncontrolled growth of the cell line (Ellerhorst-Ryan, 1997). CML has three distinct phases: the chronic phase with minimal physical symptoms, an accelerated phase with erratic cytopenias, and a phase marked by excessive myeloblasts, known as a blast crisis.
Chronic Lymphocytic Leukemia
The chronic leukemia that affects the lymphoid cell line is known as chronic lymphocytic leukemia (CLL). It is characterized by the accumulation of mature-appearing lymphocytes in the peripheral blood associated with infiltration of the bone marrow, spleen, and lymph nodes. It is important to distinguish early CLL from reactive lymphocytosis in asymptomatic patients. In reactive lymphocytosis, the cells are polyclonal and predominantly T lymphocytes, whereas in CLL they are usually B cells.
ACUTE LEUKEMIA
The acute leukemias are also a heterogeneous group of disorders. Acute myelogenous leukemia, also known as acute nonlymphocytic leukemia, arises from the myeloid stem cell. The term “acute myelogenous leukemia” is often used nonspecifically to encompass all the subgroups. In contrast, acute lymphocytic leukemia (ALL) arises from the lymphoid stem cells.
Leukemic subtypes are defined by the hematopoietic stage that was interrupted by the disease. Leukemia is not “hyperproliferative” because the mitotic rate of leukemic cells is not greater than normal white blood cells. Rather, leukemia results from an overabundance of nonfunctional white blood cells, which proliferate until there are so few normal white blood cells that the patient is overcome, often by infection, and dies.
CYTOGENETICS
Cytogenetic abnormalities, or the rearrangement, translocation, and deletion of chromosomes, take many forms. A cytogenetic evaluation at the time of diagnosis of a myeloproliferative disease enables the evaluator to classify the tissue submitted for review and enables the provider to form a prognosis. For example, in MDS, the 5q minus (chromosome 5 missing part of its longer arm) is associated with a disease course longer
than 5 years. Alternatively, if chromosome 7 is missing in MDS, the prognosis is worse, and patients tend to develop leukemia within the year (Sensenbrenner, 1995). Unfortunately, except for the presence of the Philadelphia chromosome in CML, it is difficult to judge whether a particular genetic event is associated with malignant transformation. This is because of the heterogeneous nature of chromosomal abnormalities and the apparent lack of such features in many of the people diagnosed with these disorders (Brock, 1993).
than 5 years. Alternatively, if chromosome 7 is missing in MDS, the prognosis is worse, and patients tend to develop leukemia within the year (Sensenbrenner, 1995). Unfortunately, except for the presence of the Philadelphia chromosome in CML, it is difficult to judge whether a particular genetic event is associated with malignant transformation. This is because of the heterogeneous nature of chromosomal abnormalities and the apparent lack of such features in many of the people diagnosed with these disorders (Brock, 1993).
EPIDEMIOLOGY
There were approximately 27,600 new cases of leukemia diagnosed in 1996, about equally divided between acute and chronic leukemia (American Cancer Society, 1996). Each year in the United States there are approximately 3000 new cases of MDS diagnosed (Castro-Malaspina, 1995). The incidence for these disorders combined is 9 of every 100,000 people. Leukemia is the sixth most common type of cancer in both men and women. In children, however, leukemia is the leading cause of death from disease (Sandler, 1992). Although leukemia is often thought of as a childhood cancer, it is much more common in adults: 10 times more adults than children are affected by leukemia. This seems to be an age-related phenomenon, and the incidence rises sharply for every decade after age 40 (American Cancer Society, 1996).
A few risk factors have been clearly identified in the development of myeloproliferative diseases, including chemical toxins (industrial, environmental, and iatrogenic), radiation, viruses, and congenital disorders. Table 39-1 outlines known leukemogenic pathogens.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








