Acute Renal Failure
Gail A. Breen PharmD, BCPS
Acute renal failure (ARF) is a deterioration of renal function, over a period of hours to days, resulting in failure of the kidney to perform its necessary processes, which include the elimination of nitrogenous waste products, homeostasis of fluid and electrolytes, and acid–base balance. An elevation in nitrogenous wastes is referred to as azotemia. Uremia is the clinical manifestation of azotemia and consists of nausea and vomiting, altered sensorium, pruritus, asterixis, decreased appetite, and pericarditis.
ARF is commonly defined as an increase in the serum creatinine concentration of 0.5 mg/dL when the baseline creatinine is less than 3 mg/dL, or an increase in serum creatinine of 1 mg/dL if the baseline creatinine exceeds 3 mg/dL (Rose, 1987). However, the serum creatinine level does not always rise in proportion to renal damage. Therefore, ARF can be more broadly defined as a time when the body cannot maintain adequate acid–base balance and volume status or accumulates nitrogenous wastes.
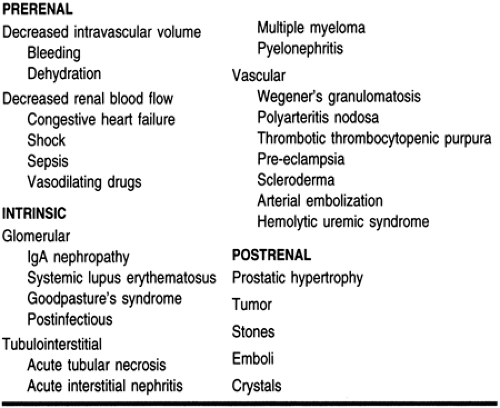
ARF is divided into three categories: prerenal (resulting from renal hypoperfusion), intrarenal (resulting from direct damage to the kidney), and postrenal (resulting from a urine flow obstruction). It is important to identify which type of renal failure a patient has, because treatment approaches vary depending on the etiology.
Risk factors for developing ARF have been defined for different patient populations and will be discussed in this chapter. Identification of patients with such risk factors is important in the prevention of ARF. Early detection and intervention is important in minimizing permanent damage to the kidneys. Despite recent advances in the medical care of these patients, the treatment of ARF continues to pose a serious dilemma for the primary care provider.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Anatomy and Physiology
Normally, people have two kidneys measuring 10 to 13 cm. Each kidney consists of approximately 2.5 million nephrons, the functional units of the kidney. The nephrons are responsible for production of an ultrafiltrate; reabsorption of electrolytes, bicarbonate, glucose, and essential amino acids; and secretion of electrolytes, medications, and other constituents of the blood that are not necessary for homeostasis.
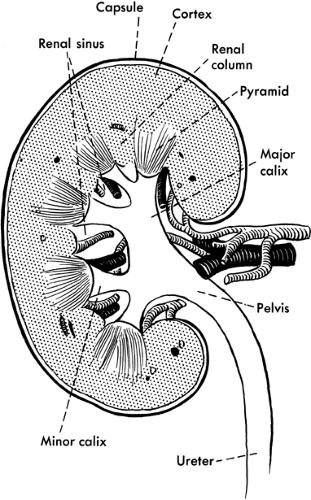
Blood flows to each kidney via the renal artery, which divides into two branches before arriving at the kidneys. Each branch divides into approximately five segmental branches, providing blood flow to their respective portions of the kidney. Each segmental branch further breaks down into smaller arteries and arterioles. Each nephron consists of a glomerulus (a vascular tuft that joins the efferent and afferent arterioles) and the collecting tubules, which consists of the proximal tubule, the loop of Henle, and the distal tubule. In the proximal tubule, 60% to 70% of the filtered load of water and solute is reabsorbed, including amino acids, glucose, and bicarbonate. Reabsorption of calcium, potassium, and magnesium occurs at the loop of Henle, as well as maintenance of an osmotic gradient necessary for the concentration of urinary solutes. The distal tubules are responsible for acid–base balance, secretion of potassium, and reabsorption of water. Figure 30-1 illustrates the anatomy of the kidney.
Pathology
Prerenal failure results from decreased renal perfusion, with or without systemic hypotension. Under normal conditions, renal blood flow is approximately 25% of the cardiac output. In states of volume depletion or major bleeding, there is a decrease in blood volume and consequently a decrease in renal perfusion. In cases of congestive heart failure and shock, there is not a decrease in blood volume but there is a decrease in blood flow to the kidneys, which also results in renal hypoperfusion. An obstruction of the renal arteries bilaterally (or unilaterally in a solitary kidney) will also decrease renal perfusion (Brenner et al, 1987). The kidney is capable of maintaining an adequate glomerular and renal blood flow in the presence of moderate reductions in renal perfusion by autoregulation. Prerenal failure results when autoregulation fails to maintain the glomerular filtration rate.
Acute intrinsic renal failure results from damage to any part of the kidney, including the small blood vessels, glomerulus, renal tubule, and interstitium. Damage to the small blood vessels may result from cholesterol emboli deposition or malignant hypertension. Damage to the glomerulus can be caused by a number of diseases, including systemic lupus erythematosus, and Goodpasture’s syndrome. Injury to the renal tubules may result from severe renal hypoperfusion or direct toxicity from exogenous and endogenous substances. Examples of exogenous nephrotoxic substances include radiocontrast dyes, aminoglycosides, and amphotericin B. Contrast nephropathy is a relatively common cause of ARF. Most contrast dyes are vasoconstrictive, and the decrease in renal blood flow can cause
ischemic nephropathy. Myoglobin, a potential endogenous nephrotoxin, is released in rhabdomyolysis and may cause acute damage to the renal tubules. Nonselective backleak of filtrate across damaged renal tubules may also result in acute tubular necrosis (ATN). Damage to the interstitium may result from infections, such as HIV and streptococci, and many drugs, including penicillin and cephalosporin antibiotics, ciprofloxacin, sulfonamides, phenytoin, and nonsteroidal anti-inflammatory agents (NSAIDs). In the case of acute interstitial nephritis, eosinophils are usually present in the blood and urine; however, eosinophils are not found in interstitial nephritis caused by NSAIDs. Symptoms of acute interstitial nephritis include fever, rashes, eosinophilia, and eosinophiluria.
ischemic nephropathy. Myoglobin, a potential endogenous nephrotoxin, is released in rhabdomyolysis and may cause acute damage to the renal tubules. Nonselective backleak of filtrate across damaged renal tubules may also result in acute tubular necrosis (ATN). Damage to the interstitium may result from infections, such as HIV and streptococci, and many drugs, including penicillin and cephalosporin antibiotics, ciprofloxacin, sulfonamides, phenytoin, and nonsteroidal anti-inflammatory agents (NSAIDs). In the case of acute interstitial nephritis, eosinophils are usually present in the blood and urine; however, eosinophils are not found in interstitial nephritis caused by NSAIDs. Symptoms of acute interstitial nephritis include fever, rashes, eosinophilia, and eosinophiluria.
Postrenal obstruction is characterized by an acute onset of anuria. The incidence of postrenal failure is low: it accounts for less than 10% of all cases of ARF (Balsov & Jorgensen, 1963). It must occur in both kidneys to cause ARF, or in one kidney if the patient has a solitary kidney. Crystal deposition from oxalate or drugs such as acyclovir or sulfonamides may cause a urinary obstruction. Malignancy, atonic bladder, or urethral stricture can cause obstruction. Benign prostatic hypertrophy, a progressive enlargement of the prostate, is a common cause of acute postrenal failure, particularly in men older than age 50 (Wood & Bosley, 1991).
ARF can also be classified based on urine output. In the absence of obstruction, urine output directly correlates with the glomerular filtration rate in patients with ARF. Anuria is defined as a 24-hour urine production of less than 50 mL. Oliguria is defined as a 24-hour urine output of 50 to 400 mL. Nonoliguria is defined as a 24-hour urine output of greater than 400 mL. Patients with nonoliguric ARF have a better prognosis than those with oliguric renal failure, probably because of the decreased severity of the insult (Corwin et al, 1987). Tables 30-1 and 30-2 outline the causes of ARF.
EPIDEMIOLOGY
ARF is more common in the hospital setting than in the community setting. For example, the incidence of ARF is 1% at the time of admission to the hospital (Kaufman et al, 1991), increases to 2% to 5% during hospitalization (Maher et al, 1989; Shusterman et al, 1987), and rises to 4% to 15% after cardiopulmonary bypass (Zanasrdo et al, 1994). However, because of the difficulty in defining and ultimately diagnosing ARF, the exact incidence is unknown.
There are a number of risk factors for ARF, including sepsis, bleeding, volume depletion, surgery, chronic liver disease, and nephrotoxic drugs. There appears to be a direct relation between the number of failed organ systems and the death rate associated with ARF (Maher et al, 1989). The mortality rate increases two- to eightfold as the number of failed organ systems increases from zero to three (Maher et al, 1989).
The mortality rate associated with ARF has improved in recent years, probably because of more aggressive nutritional therapy and earlier and improved renal replacement therapies. Before the development of dialytic therapies, the most common causes of death in patients with ARF were uremia, hyperkalemia, and volume overload. With the improvement of dialytic techniques, the most common causes of death now are sepsis, cardiovascular and pulmonary dysfunction, and the withdrawal of life-support measures (Turney, 1990; Woodrow & Turney, 1991).
DIAGNOSTIC CRITERIA
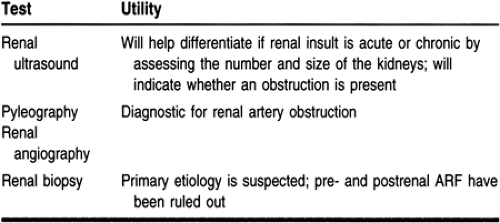
The first step in the diagnostic process is to determine whether the ARF is the result of a prerenal, postrenal, or intrinsic renal event. Prerenal failure and intrinsic renal failure from ischemia
or nephrotoxins are responsible for most episodes of ARF (Thadhani et al, 1996). Diagnostic tests that may help with the diagnosis of ARF are listed in Tables 30-3, 30-4, and 30-5.
or nephrotoxins are responsible for most episodes of ARF (Thadhani et al, 1996). Diagnostic tests that may help with the diagnosis of ARF are listed in Tables 30-3, 30-4, and 30-5.
Blood tests that help in the diagnosis and workup of ARF include serum creatinine and blood urea nitrogen (BUN); levels of both are usually elevated. The rise in creatinine may lag behind the rise in BUN and may not be detected until some time after the renal insult. The ratio between BUN and creatinine can be used to help pinpoint the cause of kidney dysfunction. The BUN/creatinine ratio is usually 10:1, but it varies depending on the etiology of ARF. An increase in creatinine kinase levels in association with a traumatic event or prolonged ischemia may be indicative of rhabdomyolysis (Kurokawa, 1983). Hypercalcemia and hyperuricemia may indicate a malignant cause; eosinophilia may point to an allergic interstitial nephritis. Tests for immunologic substances such as serum complement 3 and 4 would suggest glomerulonephritis and alert the provider to a systemic cause, such as lupus nephritis.
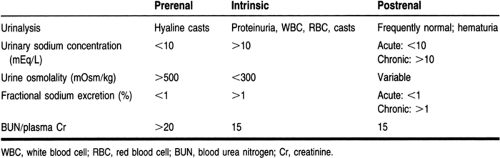
An extensive examination of the urinalysis is crucial to the diagnosis. Proteinuria and hematuria indicate a glomerular injury; glucosuria suggests a tubular injury. Microscopic examination of the urine is also important. Examples of diagnostic findings on the microscopic urinalysis include pigmented, muddy-brown granular casts, which are characteristic of tubular necrosis; red blood cell casts, which are suggestive of a glomerular injury; and white cell casts, which are indicative of interstitial nephritis.
Urine indices, including urine osmolality, urine sodium concentration, and fractional excretion of sodium, help differentiate between prerenal azotemia and ATN (Rose, 1987). The equation for calculating the fractional excretion of sodium is (Thadhani et al, 1996):
(UNa × PCr × 100)/UCr × PNa
In prerenal conditions, the concentration ability of the kidneys and the reabsorptive function of the tubules are usually intact, whereas in ATN these processes are usually lost or impaired. Because the ability of the kidneys to concentrate the urine is diminished in the setting of ATN, urine osmolality is decreased, and the urinary fractional excretion of sodium (and hence the urine sodium concentration) is increased. However, the guidelines for urine indices are not rigid. For example, early in ATN secondary to contrast nephropathy, urinary excretion of sodium is low. Concomitant diuretic administration may limit the usefulness of the fractional excretion of sodium by increasing sodium excretion (Rose, 1987).
Prerenal azotemia and ATN together account for 70% to 75% of cases of ARF in the hospital setting (Miller et al, 1978). Prerenal azotemia and ATN are often difficult to differentiate, because prerenal failure may lead to ATN. Treatment options differ between these two causes; therefore, every effort must be made to distinguish the cause. Aggressive fluid administration would be the primary treatment modality of prerenal azotemia but may be detrimental in ATN. Laboratory data, as mentioned previously and in Table 30-1, will aid in the correct diagnosis.
HISTORY AND PHYSICAL EXAM
A careful history is crucial in the initial evaluation. A history of concomitant disease states, family medical history, and medication use are all pertinent in the diagnosis. A recent history of an exacerbation of congestive heart failure, volume depletion secondary to a traumatic injury, or angiography may suggest the diagnosis of ARF.
A thorough physical exam is a necessary part in the workup of ARF. Clinical findings of thirst and orthostatic dizziness and physical findings of orthostatic hypotension, tachycardia, and dry skin turgor suggest prerenal failure secondary to volume depletion. A rash may suggest allergic interstitial nephritis. Flank pain may indicate a renal artery or vein occlusion. Digital ischemia may suggest atheroembolization.
TREATMENT OPTIONS, EXPECTED OUTCOMES, AND COMPREHENSIVE MANAGEMENT
ARF is commonly a result of iatrogenic causes, so emphasis must be placed on prevention. Risk factors for the development of ARF secondary to a number of causes have been defined, and their identification is the first step in prevention. Once risk factors are identified, different measures can be taken to avoid ARF. For example, if a patient is at risk for aminoglycoside nephrotoxicity, a different antibiotic may be used, such as a cephalosporin antibiotic with gram-negative coverage. If a patient is at risk for postoperative ARF, medical treatment may be employed, or surgery may be delayed until an underlying risk is corrected. If a patient is at risk for contrast-associated nephrotoxicity, adequate hydration and volume expansion should be implemented. Risk factors for the development of ARF are outlined in Table 30-6.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree