Critical care medicine and the development of intensive care units were initiated in response to the increased incidence of respiratory failure observed during the US polio epidemic of the 1940s, with the first critical care medicine training program established by a University of Pittsburgh anesthesiologist, Dr. Peter Safar.
The scope of CCM is vast, covering nearly every aspect of illness and injury and drawing upon knowledge from nearly every medical and surgical specialty. It is also a specialty that continues to undergo tremendous change. New technologies, equipment, and medications along with increased understanding of diseases and pathophysiology allow for the treatment of increasingly ill patients. More recently, as the economics of health care delivery have received increased attention, the focus on delivering evidence-based, cost-effective care has also increased. Because the ICU is one of the most resource-intensive areas of modern hospitals, care of patients in the ICU has been identified as an obvious target for increased efficiency.
Although the entire spectrum of critical care is beyond the scope of this chapter, widely applicable aspects of contemporary critical care are reviewed here. The first part of the chapter addresses processes of care that are applicable to most ICU settings, including both medical and surgical ICUs. The second part of the chapter provides an overview of the management for some commonly encountered diagnoses. The entire chapter focuses on evidence-based practices that may improve both patient outcomes and health care system performance in the perioperative setting.
I. Processes of Care
A. Staffing
Advances in medical and surgical therapeutics have increased the complexity of care for an aging and increasingly ill population. It has become clear that providing the best care for such a population requires a knowledge base and skill set that are highly specialized and that involving intensivists in the care of critically ill patients is desirable. Compared with low-intensity staffing models, high-intensity staffing models (i.e., an intensivist-led team or mandatory consultation of an intensivist) are associated with lower hospital and ICU mortality as well as shorter hospital and ICU lengths of stay (1).
Patient outcomes appear to be further improved by the addition of multidisciplinary providers to intensivist led teams. Examples include pharmacist participation in daily rounds, as well as the inclusion of nurses, dieticians, and respiratory therapists. These practices significantly reduce costs and medication-related adverse events and are also associated with decreased patient mortality (2).
B. Checklists
Despite the improvement in communication and information transfer that occurs with multidisciplinary teams, high stress and a massive volume of information in the ICU environment can lead to errors. Checklists have been widely implemented on ICU rounds as cognitive aids that serve as daily reminders to evaluate a limited number of interventions, preventative measures, bundles, and processes of care that can improve outcomes. Their implementation is associated with decreased mortality and ICU length of stay, and their cost is negligible (3). Considering the potential benefits and the minimal economic investment required for checklist implementation, their use is strongly recommended. In fact, many of the care processes discussed in this chapter commonly appear on checklists and should be considered for every patient, every day. Suggested content for consideration and inclusion in a daily ICU checklist is listed in Table 41-1. Content might be added or removed based on local ICU considerations.
Table 41-1 Suggested Daily Intensive Care Unit Checklist

C. Resource Management
In 2014, the Critical Care Societies Collaborative released a list of “Five Things Physicians and Patients Should Question” in critical care as part of the Choosing Wisely campaign. The campaign is designed to reduce unnecessary interventions that lack cost-effectiveness and has been supported by many medical specialties. At the top of the list is a recommendation to not order diagnostic studies (chest x-rays, blood gases, blood chemistries and counts, and electrocardiograms) at regular intervals (e.g., daily) unless there is a clear indication. Compared with the practice of ordering tests only to answer clinical questions or when doing so will directly affect management, the routine ordering of tests increases costs, does not benefit patients, and may in fact harm them. This and other efforts to minimize unnecessary interventions recognize both the financial impact medical practice decisions have on individual patients and the health care system overall. These efforts also emphasize the physician’s role in providing not just effective care, but efficient care.

Once commonplace in intensive care units, the routine, scheduled performance of diagnostic tests (e.g., daily chest x-ray) is no longer indicated due to increased costs, questionable clinical benefit, and possible patient harm.
D. Analgesia and Sedation
Pain
Pain, agitation, and delirium (PAD) are closely linked and are often experienced by critically ill patients. There are many causes of PAD related to the underlying medical conditions and also to the assessments and interventions that occur as part of care. An effective PAD management program should be tailored to each individual patient and involve steps to prevent, detect, quantify, treat, and reassess PAD. Current guideline recommendations are summarized in Figure 41-1 (4).
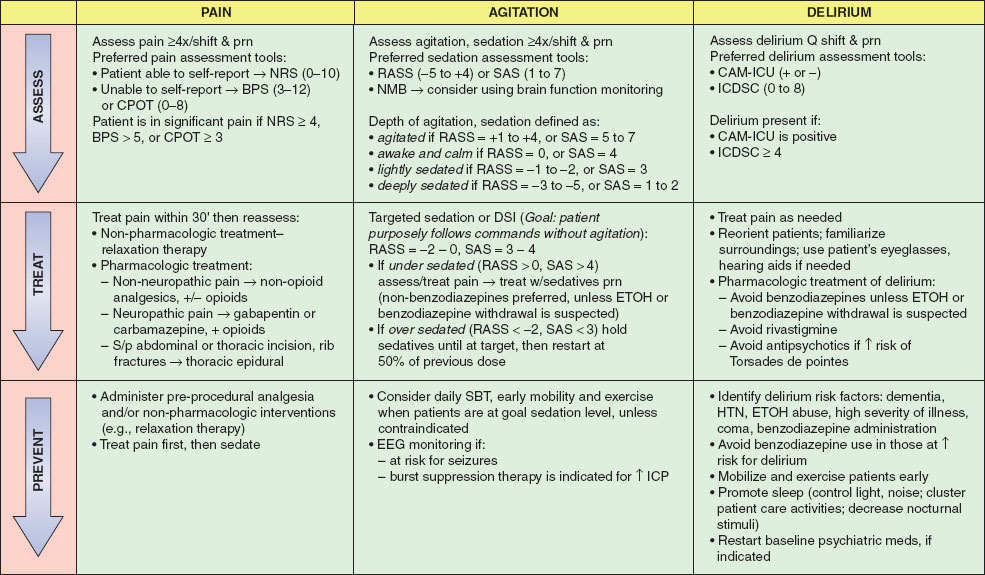
FIGURE 41-1 Pain, Agitation and Delirium Guideline Implementation. NRS, Numeric Rating Scale; BPS, Behavioral Pain Scale; CPOT, Critical care Pain Observation Tool; RASS, Richmond Agitation Sedation Score; SAS, Sedation Agitation Scale; CAM-ICU, Confusion Assessment Method for the intensive care unit; ICDSC, Delirium Screening Checklist; DSI, Daily Sedation Interruption; SBT, spontaneous breathing trial; IV, intravenous; NMB, neuromuscular blockade; ETOH, ethanol; nonbenzodiazepines, propofol (use in intubated/mechanically ventilated patients), dexmedetomidine (use in either intubated or nonintubated patients); EEG, electroencephalography; ICP, intracranial pressure; HTN, hypertension. (Reproduced from Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41:263–306, with permission.)
Opioids are the primary modality of treating moderate to severe pain in critically ill adults. However, they have undesirable side effects including nausea, constipation, respiratory depression, and alteration of mental status. When titrated to similar endpoints, there is not one opioid medication that has been shown to be superior to others. In order to reduce opioid-related side effects, nonopioid analgesics such as acetaminophen, nonsteroidal anti-inflammatory drugs, local anesthetics, ketamine, and γ-aminobutyric acid analogues may be used in conjunction with opioids. In the case of mild pain, acetaminophen or nonsteroidal anti-inflammatory drugs may replace opioids altogether. Regional anesthesia techniques may also be helpful in managing a variety of types of pain including extremity injuries, incisional pain on the torso, and rib fractures. Neuropathic pain, commonly related to diabetes and vascular disease, can be just as debilitating as nonneuropathic pain and therefore deserves equal attention. Although all these medications can play a role in the management of neuropathic pain, the γ-aminobutyric acid analogues, such as gabapentin, pregabalin, and carbamazepine, are recommended as first-line agents. In all instances, before and after assessments of pain severity should be made to guide further analgesic administration. A number of validated assessment instruments exist including the Numeric Rating Scale, Behavioral Pain Scale, and the Critical care Pain Observation Tool. It is important to note that vital signs alone should not be used to assess pain but may be a clue (i.e., pain-induced sympathetic nervous system activation) to further investigate whether pain is present.
Agitation and Delirium
A variety of factors may contribute to the development of agitation and delirium including pain, hypotension, hypoxemia, hypoglycemia, alcohol and other drug intoxication or withdrawal, sleep cycle alteration, light, noise, and mechanical ventilation. These contributing factors should be sought out and aggressively corrected. Similar to pain treatment, treating agitation and delirium effectively requires patient assessment followed by an intervention, with a subsequent reassessment to determine the effectiveness of the intervention. There are multiple validated agitation and delirium assessment tools, including the Richmond Agitation Sedation Score and the Sedation Agitation Scale for agitation and the Confusion Assessment Method for the ICU and ICU Delirium Screening Checklist for delirium. All patients should have daily screening with one of these tools. When agitation and delirium are detected, it is especially important to differentiate pain from other etiologies as appropriate treatment of pain may eliminate the need for additional sedatives. Efforts should also be made in all patients to minimize sleep interruption and to maintain a normal sleep–wake cycle. Opening the window shades, turning on lights, engaging the patient with conversation, television, and radio during the day, while turning off lights, minimizing noise, and minimizing procedures at night all help to achieve this goal.
When pharmacologic intervention is necessary, the goal is to use the smallest amount of sedative possible. Although deeper states of sedation may make patient care easier, minimal sedation is associated with improved outcomes including reduced duration of mechanical ventilation and shorter ICU stays. Pharmacologic treatment of agitation commonly involves benzodiazepines, propofol, and dexmedetomidine. Although all three agents are potentially useful for agitation treatment, benzodiazepines are associated with increased delirium and should be minimized unless alcohol withdrawal is suspected. Regardless of the agent used, unless there is a specific contraindication to stopping sedation, all patients should have a daily spontaneous awakening trial (SAT).

For mechanically ventilated patients in the intensive care unit who are recovering respiratory function, a spontaneous breathing trial (reduced ventilator support while the trachea is still intubated) is often coupled with a spontaneous awakening trial (reduced administration of sedative medications) to determine the likelihood of successful tracheal extubation.
II. Ventilator Liberation and Spontaneous Breathing Trials
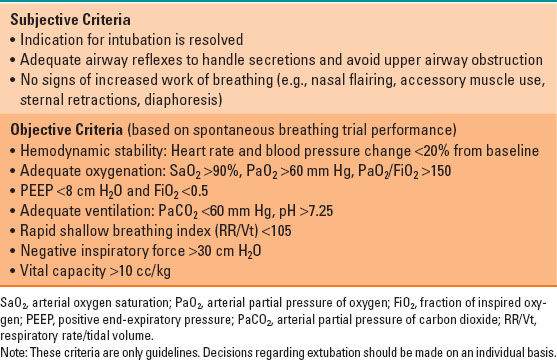
Liberation (sometimes called weaning) from mechanical ventilation is a process that requires, at a minimum, adequate oxygenation and ventilation without mechanical assistance. However, safe removal of the endotracheal tube requires patients to meet additional criteria including the ability to manage secretions and protect their airway from aspiration and obstruction.
Both objective and subjective parameters should be examined in order to determine a patient’s suitability for liberation from mechanical ventilation. Table 41-2 lists commonly used criteria for extubation. The objective criteria require the patient to undergo a spontaneous breathing trial (SBT). The SBT is a 30- to 120-minute trial of breathing with little or no assistance from the ventilator. It may be performed with a variety of techniques including T-piece trials, pressure support ventilation trials, and continuous positive airway pressure trials, although no single technique is superior to another. An SBT should be performed daily on all patients who qualify. Typically this includes mechanically ventilated patients requiring <60% fraction of inspired oxygen (FiO2) and positive end-expiratory pressure (PEEP) <8 cm H2O. When combined with a protocolized daily SAT, the daily SBT has been shown to shorten the duration of mechanical ventilation and may improve mortality.
Table 41-2 Criteria for Extubation
A. Venous Thromboembolism
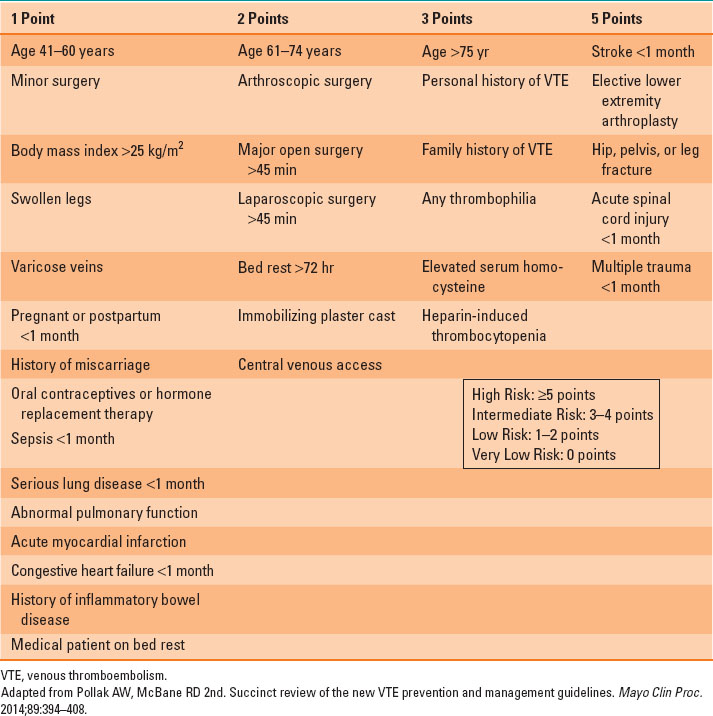
Deep venous thrombosis (DVT) and venous thromboembolism (VTE) are common problems in critically ill patients. The incidence may be as high as 30% for DVTs and 5% for pulmonary embolism (PE) depending on the population. The pathologist Rudolph Virchow was the first to describe the combination of three factors that predispose patients to venous thrombosis. The triad of hypercoagulability, venous stasis, and vascular endothelial damage bare his name, and almost all ICU patients have at least one of these risk factors. A simple scoring system for VTE risk stratification is shown in Table 41-3. Determining VTE risk is important in that it helps in choosing prophylactic therapy and in determining the level of suspicion for VTE in individual patients (5). The clinical decision of which patients to treat prophylactically and how to treat them always balances the risk of VTE with the risks of VTE prophylaxis, including heparin-induced thrombocytopenia and bleeding. It is generally agreed that high-risk patients without contraindications should receive prophylaxis with low molecular-weight heparin (LMWH). Patients with low to moderate risk should receive either low-dose unfractionated heparin (UFH) or LMWH. Patients with contraindications to LMWH or UFH may receive prophylaxis with mechanical devices (serial compression devices), and in some cases inferior vena cava (IVC) filters. But IVC filters should be reserved for the setting of high VTE risk and ongoing contraindications to anticoagulation. There is no evidence to support the routine preventive placement of IVC filters in the critically ill, including those with traumatic injuries. UFH and LMWH do not need to be routinely held prior to most surgical procedures (unless regional anesthesia is under consideration), but decisions regarding perioperative anticoagulation should be made in conjunction with the surgical team.
Table 41-3 Caprini Risk Assessment Model for Venous Thromboembolism
Although routine DVT screening studies are not recommended, asymptomatic DVTs are common. Thus, VTE should be considered in patients with nonspecific findings such as tachycardia, tachypnea, fever, asymmetric extremity edema, and gas exchange abnormalities. Compression Doppler ultrasonography is the most commonly used test for diagnosis of DVT. It has a good positive and negative predictive value. However, regularly scheduled screening compression Doppler ultrasonography is not generally recommended. The Well’s criteria and D-dimer levels, tests commonly used in the outpatient setting, have little role in critically ill patients due to their lack of specificity.
The mainstay of treatment for VTE is heparin, which should be started prior to confirmatory studies if clinical suspicion is high. The advantage of UFH over LMWH in the ICU population is its titratability and rapid reversibility, which may be desirable in patients at high risk for bleeding and in those with renal insufficiency. In patients with PE and hemodynamic instability, chemical thrombolytic therapy or mechanical thrombectomy should be considered (if not contraindicated). Although the data supporting thrombolytic therapy for treatment of PE are limited, patients with massive PE or shock are likely to benefit.
B. Nutrition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








