Coronary Artery Disease in Primary Care
Joseph J. DeRose Sr. MD
Joseph J. DeRose Jr. MD
Marielaina S. DeRose MD
Mortality from cardiovascular disease has declined significantly from the early 1970s until today (National Institute of Health, 1994). In the period from 1980 to 1988 the age-adjusted ischemic heart disease death rate for patients older than 35 years of age fell 24%, from 588.3 to 448.8 per 100,000 (American Heart Association, 1991). Despite these marked reductions, coronary artery disease (CAD) remains the number-one cause of death in the United States for both men and women. Approximately 900,000 cases of acute myocardial infarction (MI) occur annually, resulting in nearly 250,000 deaths (Farmer & Gotto, 1997). The costs of traditional present care are estimated at $8 to $10 billion annually, and 50% of patients admitted to the hospital for possible acute ischemic CAD are found to have noncardiac causes. As such, CAD remains an enormous public health problem, and its evaluation and management remain a major clinical challenge.
Knowledge of the etiology, pathogenesis, clinical manifestations, diagnosis, and treatment of CAD has grown considerably. It remains the responsibility of the primary care provider not only to recognize patients with cardiac chest pain but also to identify and treat risk factors associated with CAD in asymptomatic patients. It is hoped that preventive guidelines will reduce both the morbidity and mortality rates of this disease.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Coronary Anatomy
The right coronary artery and left coronary artery are the two major arterial trunks that originate from the aortic root to supply the myocardium. Functionally, the coronary circulation is divided into the right coronary artery, which perfuses the right ventricle; the left anterior descending branch of the left coronary artery, which supplies the anterior wall of the left ventricle and the anterior septum; and the circumflex branch of the left coronary artery, which perfuses the lateral wall of the left ventricle (Fig. 9-1).These are the three territories that clinicians frequently refer to as “triple-vessel disease” when describing coronary artery pathology.
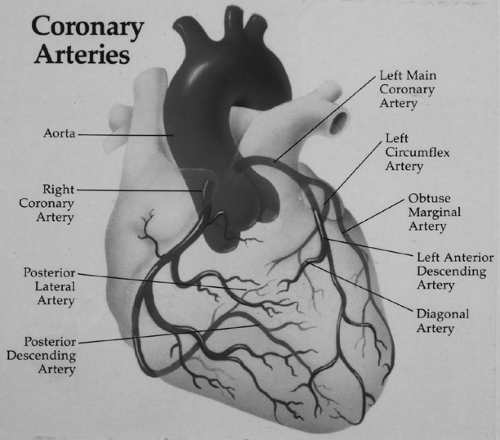 FIGURE 9-1 Coronary arterial anatomy. The three major arterial distributions include the right coronary artery, the left anterior descending artery, and the circumflex artery. |
The coronary circulation is referred to as “right dominant” when the major vessel supplying the posterior aspect of the left ventricle, the posterior descending artery, originates from the right coronary artery. In contrast, a “left dominant” coronary circulation is one in which the circumflex artery gives rise to the posterior descending artery. Seventy-five percent of patients have a right dominant circulation, 15% of patients are left dominant, and 10% of patients have a balanced coronary circulation (Kirklin & Barratt-Boyes, 1993).
The arteriosclerotic process usually affects multiple coronary arteries. Among all patients undergoing coronary angiography, 40% have all three vessels affected; approximately 30% have disease in two vessels. The main trunk of the left coronary artery has a significant stenosis in 10% to 20% of patients undergoing angiography (Gensini, 1975). The disease process usually affects the proximal portions of larger coronary arteries at or just beyond the sites of branching. When the disease is more extensive, the secondary distal branches of the larger coronary arteries may be affected, rendering them unsuitable for interventional or surgical revascularization procedures (Kirklin & Barratt-Boyes, 1993; Gensini, 1975).
Arterial Physiology
The arterial tree is more than just a series of conduits through which blood travels to the various organs. Rather, both normal and diseased arteries are capable of complex biologic processes involved in hemostasis, cytokine and growth factor secretion, permeability, metabolism of vasoactive substances, connective tissue formation, lipid metabolism, and cellular proliferation.
The normal artery is composed of three layers: intima, media, and adventitia. A single cell layer of endothelial cells lines the luminal aspect of the blood vessel and makes up the intima. Endothelial cell integrity is critical for maintaining a permeability barrier between the blood and the extracellular tissues. Endothelial cells are also responsible for providing an intraluminal nonthrombogenic surface. They secrete a wide range of vasoactive substances, including endothelial-derived relaxing factor, prostacyclin, endothelin, angiotensin-converting enzyme, and platelet-derived growth factor. A special capacity of endothelium particularly important in atherogenesis is its ability to modify lipoproteins. Low-density lipoproteins (LDLs) can be bound by LDL receptors on endothelial cells, internalized, and modified. Modified LDLs can then bind to scavenger receptors on the surface of macrophages to form foam cells, an important contributor to the atherosclerotic plaque (Krieger, 1995).
The muscular layer of the artery is termed the media and is composed of smooth muscle cells. These smooth muscle cells normally exist in a quiescent state and exhibit a contractile phenotype that is capable of altering blood vessel tone. Like endothelial cells, these smooth muscle cells exhibit LDL receptors and are capable of lipoprotein modification and presentation to macrophages. With overlying endothelial denudation or injury, medial smooth muscle cells are capable of responding to a variety of mitogens and chemoattractants. The quiescent contractile smooth muscle cell can then be stimulated to differentiate into a proliferative phenotype that can migrate into the intima, divide, and secrete extracellular matrix (Krieger, 1995; Ross, 1993).
The outermost layer of the vessel wall, the adventitia, is composed of a thin layer of collagen and fibroblasts. Although once thought to be a biologically inactive layer, it is now being recognized that adventitial fibroblasts participate in the processes of atherogenesis and arterial restenosis. Differentiation of adventitial fibroblasts into myofibroblasts can result in remodeling of the vessel wall. Adventitial myofibroblasts may serve to decrease vessel size in the process of atherogenesis and arterial restenosis, not unlike their role in wound contraction during wound healing (Gibbons & Dzau, 1994).
Process of Atherogenesis
Endothelial injury is the inciting event in the generation of luminal stenosis. Disruption of the endothelial lining exposes the circulating blood elements to the underlying thrombogenic surface of the media. Platelets readily adhere to this surface and release mitogens and growth factors. Medial smooth muscle cells are stimulated to migrate into the intima, proliferate, and secrete extracellular matrix, resulting in intraluminal stenosis. Lymphocytes are also attracted to the site of injury, resulting in continued cytokine release and antigen presentation. This response to the injury process is responsible for restenosis after angioplasty and coronary artery bypass grafting (CABG), as well as the transplant atherosclerosis seen in immunologically injured endothelium of heart transplants (Ross, 1993; Gibbons & Dzau, 1994; Badimon et al, 1993).
The cause of endothelial injury in primary atherosclerosis is most commonly secondary to hyperlipidemia (Ross, 1993; Gibbons & Dzau, 1994). Circulating lipoproteins, especially LDLs, can be taken up by endothelial cells and medial smooth muscle cells. After modification and presentation, resident macrophages scavenge the modified LDLs and oxidize them. Lipid-laden macrophages in the subendothelium are responsible for the earliest lesion of arteriosclerosis, termed the fatty streak. Grossly, this appears as an area of yellow discoloration on the luminal aspect of the artery. The fatty streak is seen as early as late childhood and young adulthood and is anatomically distributed in areas ultimately affected with progressive atherosclerosis.
Oxidation of LDLs by macrophages leads to toxic injury to the endothelium through generation of superoxide radicals. This endothelial injury, together with secretion of numerous growth factors by these activated macrophages, sets into motion the cascade of smooth muscle cell activation. The resulting lesion is a fibrous plaque composed of large numbers of intimal smooth muscle cells, collagen fibers, macrophages, and lymphocytes. Continued lipid uptake results in intracellular and extracellular accumulations of cholesterol esters. Progressive growth of the fibrous plaque results in slow stenosis of the arterial lumen and eventual occlusion.
Endothelial injury in atherogenesis may also occur in response to flow dynamics associated with hypertension, glycosylation associated with diabetes, superoxide production involved in cigarette smoking, and primary viral injury from cytomegalovirus infection (Badimon et al, 1993). Although the mechanisms of endothelial injury operating in these coronary risk factors are not clearly elucidated, the cascade of proliferation and the final pathologic pathway all remain the same.
Thrombosis and Plaque Stability
Continued injury to the endothelium results in worsening endothelial dysfunction. The permeability barrier created by the endothelium is lost, and the balance between intraluminal anticoagulant and procoagulant properties is disrupted (Loscalzo, 1992). Rupture of the endothelial lining of an atherosclerotic plaque can result in intramural hemorrhage, exposure of subintimal collagen, and intraluminal thrombosis. In contrast to the slow luminal reduction caused by the progressing atherosclerotic plaque, plaque rupture and thrombosis results in acute vessel closure. Without the time necessary for compensatory
collateral formation and angiogenesis, plaque rupture results in acute myocardial ischemia and potential myocardial cell death.
collateral formation and angiogenesis, plaque rupture results in acute myocardial ischemia and potential myocardial cell death.
Coronary Blood Flow and Myocardial Ischemia
Ischemia refers to the inadequate delivery of oxygen to the myocardium, accompanied by an inadequate removal of metabolites consequent to reduced perfusion. Myocardial ischemia is the result of an imbalance between myocardial oxygen demand and myocardial oxygen supply. The therapies for CAD are aimed at re-establishing this balance by decreasing myocardial oxygen consumption, increasing coronary blood flow, or both.
DETERMINANTS OF MYOCARDIAL OXYGEN CONSUMPTION
Cardiac energy generation is primarily aerobic, and therefore myocardial oxygen consumption is an accurate measure of total cardiac metabolism. Increases in cardiac oxygen consumption are primarily affected by changes in systolic wall tension, contractility, and heart rate (Ardehali & Ports, 1990; Rooke & Fiegel, 1984; Graham et al, 1968).
Systolic wall tension is determined by both stroke volume and systolic pressure generation. These determinants of wall tension have their clinical correlates in preload and afterload. Increases in either parameter result in a greater overall external workload and an increase in myocardial wall tension (Rooke & Fiegel, 1984). By the Laplace relation, myocardial wall tension is decreased by decreasing ventricular size and increased with ventricular dilatation.
Changes in contractility increase both systolic pressure generation and time to peak pressure generation; without significant changes in ventricular volume, these translate into increased myocardial wall tension and increased myocardial oxygen consumption (Ardehali & Ports, 1990; Rooke & Fiegel, 1984; Graham et al, 1968). Positive inotropic agents also enhance excitation–contraction coupling. Increased oxygen requirements then result from greater and more rapid calcium uptake by the sarcoplasmic reticulum. Finally, acceleration of the heart rate increases myocardial oxygen demand by increasing the frequency of tension development per unit of time and simultaneously by increasing contractility (Ardehali & Ports, 1990; Rooke & Fiegel, 1984). Myocardial ischemia is the result when any of these determinants cause increased myocardial metabolic demands without concomitant regulation of oxygen delivery.
DETERMINANTS OF MYOCARDIAL OXYGEN DELIVERY
Coronary blood flow is the major determinant of myocardial oxygen delivery. Perfusion of the coronary arteries occurs primarily during diastole because of myocardial compression of the intramyocardial and subendocardial arterioles during systole. Approximately 80% of coronary flow to the left ventricle and 50% of flow to the right ventricle occur during diastole. Coronary perfusion pressure, therefore, is determined by both mean aortic pressure and left ventricular end-diastolic pressure.
Autoregulation of the coronary circulation via neurohumoral mechanisms serves to maintain coronary flow fairly constant over a wide range of perfusion pressures. In the presence of fixed coronary stenoses, however, autoregulation cannot further increase regional blood flow to accommodate increases in myocardial oxygen demand. Coronary vessels with fixed atherosclerotic lesions are maximally dilated to maximize distal flow in the setting of luminal obstruction. With further metabolic demands, autoregulation is not possible and regional myocardial ischemia with resultant myocardial dysfunction occurs. Furthermore, in the setting of atherosclerosis, endothelial dysfunction results in impaired ability to generate vasoactive substances such as endothelial-derived relaxing factor and prostacyclin. This results in further impairment of smooth muscle relaxation as well as a loss of platelet aggregation inhibition.
EPIDEMIOLOGY AND RISK FACTORS
Risk factor assessment and modification are an integral part of the evaluation and treatment of patients with both known and suspected CAD. Unfortunately, risk factors are often misinterpreted as either necessary or sufficient causes of disease. The primary care provider must be mindful of the fact that risk factors represent associations that may or may not be causal. Risk factors can be divided into modifiable and nonmodifiable categories (Table 9-1). This characterization has clinical implications: only the former can be targeted for preventive measures. However, patients with strong nonmodifiable risk factors may warrant greater intensity of risk factor management because of an increased risk of CAD.
Nonmodifiable Risk Factors
AGE, GENDER, AND FAMILY HISTORY
CAD increases linearly with age. The prevalence of CAD is greater in men than women until age 75, at which point it equalizes. The incidence of CAD in persons younger than 55 years of age is three to four times greater in men than women.
The National Cholesterol Education Program defines family history of premature CAD as MI or sudden death in a father or first-degree male relative less than 55 years of age or in a mother or first-degree female relative less than 65 years of age (Summary of the Second Report of NCEP, 1993). Family history is one of the most powerful determinants of CAD independent of other risk factors (Rissanen, 1979; Friedlander, 1994;
Hamstn & De Faire, 1987). Siblings of patients with CAD have an increased risk of dying (5.2 times higher) compared to a control population (Rissanen, 1979). The presence of this very strong nonmodifiable risk factor should prompt the physician to treat aggressively any concomitant risk factors in such a patient.
Hamstn & De Faire, 1987). Siblings of patients with CAD have an increased risk of dying (5.2 times higher) compared to a control population (Rissanen, 1979). The presence of this very strong nonmodifiable risk factor should prompt the physician to treat aggressively any concomitant risk factors in such a patient.
SOCIOECONOMIC FACTORS
A variety of psychosocial factors are associated with the development of CAD in the Western world. Social factors such as lower educational level and economic insecurity are associated with increased cardiac risk (Berkman et al, 1992; Kaplan & Keil, 1993). There is an inverse correlation between educational level and the age-adjusted mortality rate from CAD. Neither risk factor modification nor decreased MI rates have been uniform across all socioeconomic groups. The mechanism of increased risk among certain socioeconomic groups may be indirectly related to poor participation in a strict risk factor modification program. Some authors believe that the higher the level of education and socioeconomic status, the greater potential for lifestyle modification.
TEACHING AND SELF-CARE
Modifiable Risk Factors
LIPIDS
There is a well-established relation between total blood cholesterol and CAD, with a graded risk down to a cholesterol level of 180 mg/dL (Stamler et al, 1993a; Neaton & Wentworth, 1992). LDLs appear to be most strongly associated with the development of CAD. It has been estimated that with every 1% difference in LDL cholesterol, there is a 2% to 3% difference in the risk for CAD (Summary of the Second Report of NCEP, 1993). The National Cholesterol Education Program has stratified CAD risk according to LDL cholesterol level (Table 9-2) (Summary of the Second Report of NCEP, 1993).
The LDL cholesterol level increases with age and weight. Diets high in saturated fats and cholesterol similarly cause progressive increases in serum LDL concentrations. Conditions such as hypothyroidism, nephrotic syndrome, liver disease, and estrogen deficiency can also be accompanied by increases in serum LDLs. As referred to earlier, oxidative modification of LDLs plays an important role in the accelerated atherogenic process (Berliner et al, 1995; Morris et al, 1994).
There is a strong inverse epidemiologic association between high-density lipoprotein (HDL) cholesterol, and CAD. The Adult Treatment Panel II classified an HDL cholesterol level of less than 35 mg/dL as low and considered the presence of a high HDL cholesterol level to be a negative risk factor for CAD (Summary of the Second Report of NCEP, 1993). For every 1 mg/dL decrease in HDL cholesterol, the relative risk for CAD increases by 2% to 3% (Gordon et al, 1989). HDL cholesterol levels are affected by genetics, tobacco use, obesity, and physical activity. There are no clinical trials that indicate a decreased risk of coronary disease secondary only to an increase in HDL cholesterol. This is most likely because lifestyle and dietary modifications affect multiple lipids simultaneously.
The relation between triglycerides and CAD remains controversial. A 1992 Consensus Development Conference defined a triglyceride level of 200 to 400 mg/dL as borderline high, 400 to 1000 mg/dL as high, and more than 1000 mg/dL as very high (NIH Consensus Development Panel, 1993). In univariate analysis, triglycerides predict coronary disease but lose their power when other lipids (especially HDL cholesterol) are factored into a multivariate analysis. It has been suggested that HDL and triglycerides should not be considered separately when evaluating cardiac risk. Low HDL levels are usually associated with high triglycerides because of the transfer of a cholesterol ester between HDL particles and triglyceride-rich lipoproteins. Lifestyle modifications including weight loss, dietary restriction, decrease in alcohol consumption, smoking cessation, and increased physical activity all decrease triglyceride levels. The reduction in triglyceride levels is associated with the greatest risk reduction in patients with the highest risk—those with low HDL or elevated LDL cholesterol levels.
Although LDL levels are directly associated with CAD, the combination of elevated LDL cholesterol, elevated triglycerides, and low levels of HDL cholesterol places the patient in the highest lipid risk stratification category (Manninen et al, 1992; Assman & Schulte, 1992).
DIABETES
Both insulin-dependent diabetes mellitus and non-insulin-dependent diabetes mellitus are major risk factors for the development of CAD. The National Diabetes Data Group defined diabetes as a fasting blood glucose level of more than 127 mg/dL (American Diabetes Association, 1997). Atherosclerosis accounts for 80% of all deaths related to diabetes, and 25% of all heart attacks in the United States occur in persons with diabetes (Diabetes Control & Complications Trial, 1993; Getz, 1993; Schwartz et al, 1992; Stamler, 1987; Butler et al, 1985). The First National Health and Nutrition Examination Survey (NAHANES I) revealed that age-adjusted death rates for men and women with diabetes were twice those seen in persons without diabetes, with 75% of the excess mortality attributed to CAD (Kleinman et al, 1988). The pathogenesis of atherosclerosis in persons with diabetes is multifactorial because there are multiple metabolic derangements in persons with diabetes, including hyperglycemia, insulin resistance, dyslipidemia, and increased platelet aggregation.
The pathogenic role of hyperglycemia is well supported. Observational studies have shown that better metabolic control lowers vascular risk in patients with insulin-dependent diabetes mellitus, but this has not been clearly shown in non-insulin-dependent diabetes mellitus (Diabetes Control & Complications Trial, 1993; Reichard et al, 1993). The secondary complications of diabetes are additive in the risk of atherosclerosis. The development of nephropathy leads to elevated blood pressure and dyslipidemia, which together compound the patient’s cardiac risk.
HYPERTENSION
Hypertension is a major modifiable risk factor. Elevated shear stress and increased myocardial oxygen demand caused by hypertension have been implicated in atherogenesis and arterial injury. Nearly 50 million adult Americans suffer from hypertension, and the level of risk associated with elevated blood pressure varies substantially with gender, race, and age. Elderly people have a greater risk of cardiovascular events at every level of hypertension.
There is a continuous relation between both systolic and diastolic pressures and cardiovascular disease (Joint National Committee, 1993; Stamler et al, 1993b; MacMahon et al, 1990). A meta-analysis of several large prospective studies demonstrated a linear relation between hypertension and CAD, with a relative risk approaching three times the risk at the highest levels of blood pressure (MacMahon et al, 1990).
The risk of hypertension cannot be taken in isolation. It frequently coexists with other risk factors, including physical inactivity, obesity, alcohol use, hyperlipidemia, diabetes, and smoking. The presence of these CAD risk factors appears to be both causal and additive for hypertension.
TOBACCO
Cigarette smoking is an independent risk factor for cardiovascular disease. Several independent studies have demonstrated that smoking increases the cardiovascular mortality rate by 40% to 60% (Reducing the health consequences of smoking, 1989; Rigotti & Pasternak, 1996). Nicotine accelerates the process of atherosclerosis by a variety of mechanisms. Inhalation of cigarette smoke produces a transient and reversible prothrombotic increase in fibrinogen levels, increases platelet aggregation, and decreases the ability of endothelial cells to produce or release prostacyclin. Nicotine is also a potent agonist for the adrenergic nervous system, resulting in increased coronary tone and vasoconstriction. The enhanced vasoconstriction results in an imbalance between oxygen supply and demand and has been associated with silent myocardial ischemia (Deanfiels et al, 1986).
Tobacco smoke may augment cardiovascular death from other risk factors. Cigarette smoking adversely affects lipid profiles. Heavy smokers have lower levels of HDL cholesterol and higher levels of LDL cholesterol and triglycerides (Migas, 1988). Smoking has a variable effect on blood pressure. The acute inhalation results in a rise in blood pressure. Epidemiologic studies have shown that chronic smokers tend to have lower blood pressures than nonsmokers, possibly secondary to lower body weight (Green et al, 1986). The Hypertension Detection and Follow-up Program demonstrated that smokers had twice the mortality rate of nonsmokers (Langford et al, 1986). The incidence of MI is definitely diminished after smoking cessation. The risk reduction occurs early and may be demonstrated 12 months after cessation.
POSTMENOPAUSAL STATUS
CAD presents later in women than in men. Endogenous estrogen protects premenopausal women from coronary disease. Estrogen replacement therapy (ERT) confers a 50% reduction in the risk of developing CAD (Barrett-Conner & Bush, 1991). Estrogen favorably affects both HDL and LDL cholesterol. However, the beneficial effects of estrogen appear to be the result of more than just its improvement in the overall lipid profile. The nonlipid cardioprotective mechanisms of estrogen include direct actions on vessel walls, estrogen-associated calcium antagonist effects, estrogen-associated antioxidant effects, and estrogen-induced genetic changes. The initial clinical presentation of CAD is reduced by 30% to 70% in users of ERT (Barrett-Conner & Bush, 1994; Bush & Korenman, 1990). The Lipids Research Clinic Follow-Up Study demonstrated that after 9 years, estrogen users had a 64% lower risk of coronary death than nonusers (Bush et al, 1987). This was still significant after adjustments for known CAD risk factors, and 50% of the protection was afforded by estrogen-induced increases in HDL levels.
All studies on ERT have been observational in design, raising many questions regarding unrecognized biases that can account for the observed protective effect of estrogen. Some such biases include whether healthier women may be more likely to be prescribed estrogen or whether estrogen users may be more likely to participate in all prevention therapies. Proof of the protective benefit of estrogen awaits the results of prospective randomized clinical trials to answer these questions. One such study is the Women’s Health Initiative, which is a long-range study enrolling more than 165,000 women. Preliminary results from this study are expected over the next several years.
PHYSICAL ACTIVITY
The role of physical activity in the prevention of CAD remains controversial. The exact mechanism of protection is multifactorial. Exercise is important in maintaining ideal body weight, muscle mass, and normal blood pressure as well as optimizing lipid levels. Regular aerobic exercise decreases both systolic and diastolic pressures, improves the myocardial oxygen supply/demand ratio, lowers triglycerides, raises HDL levels, and decreases platelet aggregation.
The greatest risk reduction is achieved in nonactive and moderately active persons. Intense exercise is associated with decreases in total and LDL cholesterol and increases in HDL cholesterol (Seiler et al, 1988). Patients who exercise regularly have a decreased incidence of sudden death, although sedentary patients who begin an exercise regimen may be at increased risk for acute MI or malignant ventricular arrhythmia. Therefore, a thorough physical examination is recommended for people older than 40 years before initiating a vigorous exercise program. Likewise, younger patients with hypertension, diabetes, and other associated CAD risk factors should undergo a thorough assessment before undertaking an aggressive exercise regimen, including a baseline electrocardiogram (ECG). A history of angina or an abnormal ECG result should prompt further diagnostic testing before initiating an intensive exercise program.
OBESITY
The definition of obesity is arbitrary, but it may be defined as an increase of 120% above ideal body weight for height. There is a linear relation between body mass and cardiovascular mortality (Manson et al, 1995). The precise role of obesity as an independent risk factor remains unclear. The increased risk of obesity with CAD is chiefly related to its close association with other risk factors. Obesity has a direct relation with all CAD risk factors except smoking. Obesity is positively correlated with hypertension, hypertriglyceridemia, and hyperinsulinemia and negatively correlated with HDL.
Central obesity is quantified as the waist/hip ratio and has been shown to increase CAD risk. An elevated waist/hip ratio has been associated with hypertension, hypercholesterolemia, elevated levels of fibrinogen, and hypertriglyceridemia. In men, the development of CAD is correlated with the abdominal distribution of fat independent of obesity. In women, abdominal fat deposition constitutes a greater risk than obesity. The waist/hip ratio appears to be a more significant predictor than the total degree of obesity. A waist/hip ratio of less than 0.9 for men and less than 0.8 for women is desirable (Freedman et al, 1990).
ALCOHOL
The effect of alcohol on coronary disease is dichotomous. Moderate consumption (one to three drinks per day) results in a 40% to 50% reduction of CAD compared to abstinence (Gaziano et al, 1993; Yano et al, 1977). However, excessive consumption results in an overall increased risk (Shaper et al, 1988). Alcohol increases the HDL cholesterol level, which accounts for half of the reduction in MI. However, no beneficial effect of alcohol has been demonstrated in angina pectoris (Yano et al, 1977). Its adverse effects include the development of alcoholic cardiomyopathy, hypertension, and cardiac arrhythmias.
TYPE A PERSONALITY
The most notorious psychosocial factor associated with CAD is the “Type A personality.” Type A people are characterized as highly competitive, ambitious, and in constant struggle with their environment. There is an increased incidence in the development of angina pectoris but no subsequent increase in fatal cardiac events (Eaker et al, 1989; O’Connor et al, 1995). The increased rate of stable angina could not be explained by the presence of other cardiac risk factors. However, there are no data available that have clearly proven that this behavior itself, nor any modifications, changes the overall cardiac risk.
SERUM HOMOCYSTEINE
A newer, independent risk factor for atherosclerosis is an elevated plasma level of the amino acid homocysteine. A plasma homocysteine concentration of more than 15 μmol/L is referred to as hyperhomocysteinemia. Such elevated homocysteine levels can induce pathologic changes in the arterial wall, with a subsequent increased risk for CAD, peripheral vascular disease, cerebrovascular disease, and venous thrombosis. There is increasing evidence that homocysteine may affect the coagulation cascade and the resistance of the endothelium to thrombosis (Nygard et al, 1997). It may also interfere with the vasodilator and antithrombotic effects of nitric oxide. Although several epidemiologic studies have established the relation between total homocysteine level and CAD, studies are still ongoing to determine whether lowering homocysteine levels can prevent vascular occlusive events.
DIAGNOSTIC CRITERIA
The diagnosis of CAD is based on a history of chest pain or anginal equivalent pain together with diagnostic studies that demonstrate either functional or anatomic coronary obstruction. The diagnosis of MI is based on history, ECG changes, and myocardial fraction of creatine kinase. If two of these three criteria are positive, then the diagnosis of evolving MI is made. New wall motion abnormalities on echocardiography may also be used as soft criteria for recent myocardial injury.
HISTORY AND PHYSICAL EXAMINATION
History
The diagnosis of CAD is based on a careful, skillful clinical history. Within the current atmosphere of health care cost containment, a concisely focused outlined history will obviate the need for more costly testing.
Typical stable angina pectoris is described as a viselike, constrictive, crushing, or squeezing type of retrosternal chest pain induced by exertion. In some patients the quality of discomfort is described as mild pressure or substernal burning. In each instance the symptoms reach their maximal intensity in a few minutes and then dissipate with the cessation of exercise. Typical angina pectoris is relieved within minutes of rest or by using nitroglycerin. The response to nitroglycerin usually occurs within 3 to 5 minutes and can be a very useful diagnostic test. Nonetheless, it has been clearly established that esophageal pain and other syndromes may also respond to nitroglycerin.
Typical angina may be induced by exertional activities such as walking against the wind, climbing stairs or hills, vigorous arm work, and sexual intercourse. The discomfort can likewise be incited by emotional stress (panic, fear, anger, or anxiety), or it may follow a heavy meal. Anginal pain may even occur nocturnally after lying down (angina decubitus) secondary to increased ventricular filling pressure. The duration of discomfort and its cessation are reproducible for each typical anginal syndrome.
Although the area of discomfort is classically retrosternal, radiation is very common. The regions of discomfort may manifest themselves anywhere between the mandible and the epigastrium. Discomfort can radiate to the shoulders, the jaw, the ulnar surface of the left arm, the right arm, and the outer surface of both arms.
Symptoms of myocardial ischemia other than typical anginal discomfort, such as dyspnea with minimal exertion, fatigue, and faintness, are referred to as anginal equivalents. These symptoms are common in the elderly and in patients at high risk for coronary heart disease. The symptoms may be caused by elevation of the left ventricular filling pressure and despite a normal ECG should alert the provider to probable severe ischemic heart disease.
Stable angina is a predictable pattern of chest discomfort with a similar degree of severity and classic precipitating factors occurring either recently or over several months. It maintains a constant threshold in severity and relief over time. The corresponding lesion is usually a stable, fixed atherosclerotic lesion that is flow-limiting only when myocardial metabolic demands reach a threshold.
Unstable angina refers to angina of recent onset, intensifying in nature with a lower level of exertion or occurring nocturnally without immediate relief by rest or with nitroglycerin. The underlying coronary lesion in this emergent situation is usually a critical stenosis with either acute coronary vasospasm or thrombus formation, resulting in intermittent or permanent vessel occlusion. This unstable pattern requires more aggressive attention and evaluation because 20% will progress to MI within a 3-month period.
Variant angina, originally described by Prinzmetal, refers to chest pain occurring almost exclusively at rest, not precipitated by physical exertion or emotional stress. It is associated with electrocardiographic ST-segment elevation. It has been demonstrated that variant angina is related to coronary artery spasm with subsequent myocardial ischemia. In addition, it may be associated with MI, ventricular tachycardia, and ventricular fibrillation as well as sudden death (Mark et al, 1984).
Finally, silent myocardial ischemia can occur in patients with angina at rest, unstable angina, and chronic stable angina. It has been clearly established that patients who are at high risk for CAD can be evaluated by 24-hour double-channel Holter monitoring or stress testing for silent but significant electrocardiographic ST-segment changes (Cohn, 1996). Prognostically, this may be the only way to limit sudden death from MI as well as to identify and avoid an initial or subsequent MI.
Other conditions that should be included in the differential diagnosis of angina include common painful esophageal reflux, achalasia, esophageal spasm, peptic ulcer, and biliary colic (Table 9-3). Chest wall discomfort with localized pain, tenderness, and swelling of the costal cartilages, described in 1931 as Tietze’s syndrome, is a common additional differential diagnosis. Cervical radiculitis, chest wall spasm, and severe pulmonary hypertension associated with chest pain on exertion may all simulate anginal pain. Pulmonary embolism, acute pericarditis, mitral valve prolapse, and herpes zoster can all be included in the differential diagnosis. Although all of these can in most cases be readily distinguished from angina by a detailed history and a comprehensive physical examination, the physician must ensure that chronic coronary heart disease does not simultaneously exist with noncardiac disease.
Physical Examination
Although patients with chronic CAD and stable angina are often found to have an entirely normal physical examination, it is the responsibility of the primary care provider to perform a diligent and complete physical examination in all patients. The primary care provider can often identify useful clues to the diagnosis of CAD and identify patients who are at the highest risk for this disease.
The blood pressure may be chronically elevated. A diagonal earlobe crease may be identified that has had some correlation with CAD (except in Native American Indians and Asians) (Fig. 9-2). In the young, the diagonal earlobe crease is unilateral and becomes bilateral with advancing age (Tranchesi et al, 1992). Ophthalmologic examination may reveal a corneal arcus (Fig. 9-3). The size of the corneal arcus positively correlates with
age, levels of total cholesterol, and levels of LDL (Winder, 1983). The retina may reveal arteriolar atherosclerotic hypertensive or diabetic changes. The skin may demonstrate xanthelasma, which may be promoted by increased levels of triglycerides and a relative deficiency of HDLs (Winder, 1983).
age, levels of total cholesterol, and levels of LDL (Winder, 1983). The retina may reveal arteriolar atherosclerotic hypertensive or diabetic changes. The skin may demonstrate xanthelasma, which may be promoted by increased levels of triglycerides and a relative deficiency of HDLs (Winder, 1983).
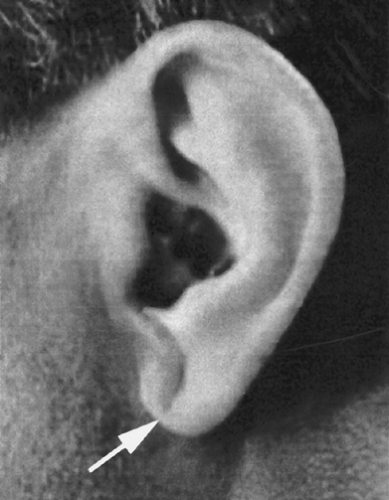 FIGURE 9-2 Photograph of diagonal earlobe crease, which may have a correlation with coronary artery disease. |
Of utmost importance in the general physical examination is to check all arterial pulses and search for abnormalities in the venous system. The association between peripheral vascular disease and coronary heart disease is well documented. This is not confined only to patients with symptomatic disease, clinically overt peripheral vascular disease, or carotid artery disease, but is also seen in asymptomatic, ultrasonically proven, hemodynamically significant obstructive arterial disease. Examination of the patient’s venous system can reveal previous venous CABG.
The cardiac examination may be most helpful during an episode of symptomatic angina because ischemia may produce transient left ventricular dysfunction with a third heart sound and bibasilar pulmonary rales. A softened mitral component of the first heart sound and paradoxical splitting of the second heart sound may occur during an anginal attack. A mid systolic click followed by a late systolic murmur may occur in patients with CAD related to transient papillary muscle dysfunction, with alteration in the alignment of the papillary muscles.
DIAGNOSTIC STUDIES
Noninvasive Testing
ELECTROCARDIOGRAPHY
Fifty percent of patients with chronic stable angina have normal ECG tracings at rest, but they may have severe CAD. However, the 12-lead ECG during a provoked episode of substernal chest pain or during an attack of angina may reveal downward or horizontal sloping depression of the ST segment, T-wave peaking, or inversion. These ECG changes accompanied by chest discomfort may signal the possibility of coronary stenosis.
EXERCISE ELECTROCARDIOGRAPHY
Exercise treadmill ECG has been the most studied noninvasive test for the evaluation of CAD in both men and women. Stress testing increases overall cardiac work and heart rate. This increased work results in an increase in myocardial oxygen demand and a subsequent requirement for increased oxygen delivery via an increase in coronary flow. If narrowed or obstructed lesions prevent the required increase in coronary blood flow, chest pain or ECG changes may arise. In both sexes the exercise ECG is most accurate in patients who are able to exercise to 85% of their maximal predicted heart rates (Severi & Michelassi, 1991). However, this goal can be modified based on the characteristics of the patient being evaluated. A young patient with a low risk for CAD will have a much more sensitive result should he or she reach the maximal heart rate target or exercise to exhaustion. An older, high-risk patient, on the other hand, may reveal clinically meaningful information at lower cardiac workloads.
Criteria for determining a positive ECG stress test vary among different institutions. Depression or downsloping of the ST segment 0.08 seconds after the J point of the ECG is the typical ischemic change sought as a positive result (Fig. 9-4). However, the degree of ST-segment depression defined as positive can range anywhere from 0.5 mV to 2 mV. Using the lower end of this range will produce a test that is more sensitive but less specific, whereas the higher end produces a result that is less sensitive but more specific. Most centers use a 1-mm ST depression as a criterion for positivity. If typical chest discomfort occurs during the test associated with an ST depression of more than 1 mm, the predictive value for the detection of CAD is 90% (Wilson et al, 1991). ST depressions of more than 2
mm in the setting of typical chest discomfort are virtually diagnostic of CAD.
mm in the setting of typical chest discomfort are virtually diagnostic of CAD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree