I. INTRODUCTION: Coronary artery disease (CAD) is the most common type of heart disease and is the leading cause of adult morbidity and mortality in the United States, accounting for ∼17% of deaths. The accumulation of atheromatous plaques within coronary arteries over time results in ischemia and/or infarction of the myocardium. Medical treatment focuses on identifying at-risk patients and preventing sequelae through risk modification. The major risk factors of CAD are hypertension (HTN), diabetes mellitus (DM), smoking, dyslipidemia (high LDL or low HDL), age above 45 (for men), age above 55 (for women), obesity or metabolic syndrome, physical inactivity, elevated homocysteine levels, and a family history of CAD. African American, Mexican American, and Native American patients also tend to have higher incidences of CAD. Aggressive risk factor modification and coronary interventions with stenting have resulted in marked improvements in the outcomes of these patients.
A. Definitions
1. Angina pectoris is substernal pressure or pain that occurs at rest or with physical or emotional stress, lasting up to 10 minutes. It can radiate to the back, jaw, arm, or shoulder, usually on the left. Angina usually reflects compromise of at least one epicardial coronary artery and implies ischemia, but not necessarily myocardial infarction (MI). Angina is the initial manifestation in 50% of patients with ischemic heart disease. Symptoms also include nausea, vomiting, diaphoresis, and shortness of breath. Angina may present in valvular heart disease, hypertrophic cardiomyopathy, or uncontrolled HTN. The Canadian Cardiovascular Society Classification System grades angina from Class I (ordinary physical exertion does not cause angina) to Class IV (angina with minimal exertion or at rest).
a. Stable angina demonstrates a pattern that has not changed in frequency, duration, or ease of relief for several months. Predictable symptoms present with exertion and abate with rest. A fixed coronary atheroma with a fibrous cap is generally the culprit lesion.
b. Variant (Prinzmetal’s) angina occurs at rest, is often worse in the morning, lasts several minutes, and is accompanied by transient ST-segment elevation and/or ventricular dysrhythmias. It can be induced by exercise and stems from vasospasm in a coronary artery that may not have significant atheromatous disease. Smoking is a major risk factor. Hyperventilation, hypocalcemia, cocaine, pseudoephedrine, and ephedrine have also been implicated.
2. Acute coronary syndromes (ACS) are three conditions associated with acute myocardial ischemia secondary to poor myocardial perfusion: unstable angina (UA), non–ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI).
a. Unstable angina indicates ischemia, has a recent onset (1–2 months), has increasing frequency and intensity, and recurs at progressively lower levels of stress, even at rest. The prognosis is poor, as 10% of patients have significant disease of the left main coronary artery and approximately 20% of patients will suffer an acute MI within 3 months. Plaque rupture, platelet aggregation, thrombosis, and vasospasm are the underlying causes.
b. NSTEMI indicates more severe myocardial ischemia in the setting of suggestive angina, resulting in a release of cardiac biomarkers. NSTEMI presents with ST-segment depressions or prominent T-wave inversions. The management of NSTEMI focuses on improving oxygen (O2) supply and reducing demand in at-risk myocardium, thus preventing progression of damage. It must always be borne in mind that a nondiagnostic electrocardiogram (ECG) does not rule out MI.
c. STEMI is defined by characteristic symptoms of myocardial ischemia with associated ST-segment elevation and subsequent release of cardiac biomarkers. There is severe, possibly irreversible damage to the myocardium, with the appearance of a new ST-segment elevation at the J-point in at least two contiguous leads ≥ 2 mm (0.2 mV) in men or ≥1.5 mm (0.15 mV) in women in leads V2–V3 and/or ≥1 mm (0.1 mV) in other contiguous leads. New or presumably new LBBB has traditionally been considered a STEMI equivalent; however, recent evidence finds that most patients with chest pain and a “new” LBBB do not have a STEMI, calling into question the validity of this practice. Left ventricular hypertrophy, hyperkalemia, pericarditis, early repolarization, or paced rhythms can complicate the diagnosis. Treatment focuses on timely reperfusion.
3. Noncardiac chest pain is not related to coronary ischemia, but may be life threatening. Aortic dissection, pulmonary embolism, and pneumothorax must be ruled out immediately. Diagnosing chest pain as cardiac or noncardiac can be challenging, and cardiac risk factors, past medical history, physical exam, and radiologic and laboratory results must be considered. Gastrointestinal and esophageal pathology, pleuritic or costochondritic causes, and recent physical or emotional stress are frequent causes of noncardiac chest pain.
4. Perioperative MI is one of the most significant threats in major noncardiac surgery. Patients who suffer MI after surgery have a 15% to 25% risk of in-hospital death and a significant increase in 6-month morbidity and mortality. While there is controversy over accepted definitions of perioperative MI, the incidence may be up to 6% in patients with CAD. While an MI is normally diagnosed on the basis of three criteria (chest pain, biomarker levels, ECG changes), a perioperative MI may be obscured by pain or pain-control techniques to the point of being “silent.” A high index of suspicion is critical, and increased consideration should be given to the ECG and cardiac enzymes.
B. Pathophysiology
1. Myocardial O2 supply–demand balance. The myocardium has the highest O2 consumption per tissue mass of all human organs. At rest, the myocardial O2 extraction ratio is near maximum at 70% to 80% leaving minimal O2 extraction reserve for increased demand. During exertion, increased O2 demand must be met with an increase in O2 delivery. Myocardial ischemia and infarction occur when O2 demand exceeds O2 delivery.
a. Myocardial O2 supply is determined by the following:
1. Coronary blood flow is determined by the aortic root pressure at the beginning of diastole minus the resistance of transmural pressure. As transmural resistance is low in diastole, myocardial blood flow is higher during this period. Tachycardia, which minimizes diastolic time, can thus induce ischemia. Normal coronary arteries compensate by sympathetically or metabolically mediated dilation, which can increase blood flow four- to fivefold during exercise or stress. However, stenosis can reduce ability of the coronary artery to dilate and thus limit O2 supply downstream. Polycythemia, hyperviscosity, and sickle cell disease may further compromise coronary blood flow.
2. O2 content is dependent on hemoglobin (Hgb) concentration and saturation with O2 (SaO2), and to a lesser extent on dissolved O2 concentration (see Chapter 2). An ideal Hgb level is not known, but compensation through increased cardiac output (CO) occurs with anemia. Hyperbaric oxygen therapy may be useful to augment O2 supply in patients with severe anemia or ACS.
b. Myocardial O2 demand is influenced by the following:
1. Ventricular wall tension (T), which according to Laplace’s law is T = PR/2h, where P is transmural pressure, R is ventricular radius, and h is wall thickness. Ventricular dilation and increased afterload both increase wall tension and myocardial O2 demand. Concentric hypertrophy can decrease wall tension, but also increases the amount of myocardium to be perfused.
2. Heart rate (HR) increases O2 demand by increasing O2 consumption. Tachycardia shortens diastole and limits coronary perfusion in atherosclerotic vessels thereby limiting O2 supply. Hyperthyroidism, sympathomimetics (e.g., cocaine), or anxiety can increase HR and O2 demand.
3. An increase in contractility, which is the intrinsic property of the myocyte to contract against a load, increases O2 demand. Positive inotropes (e.g., epinephrine, digoxin) increase the O2 demand of myocardium that may already be at risk.
2. Etiologies of myocardial O2 demand imbalance. More than 90% of myocardial ischemia and infarction result from atherosclerosis. Accordingly, most perioperative MIs stem from abrupt, unpredictable occlusion (partial or complete) secondary to acute atherosclerotic plaque rupture. Other causes include coronary vasospasm or thromboembolism, stent thrombosis, vasculitis, trauma, valvular heart disease (e.g., aortic stenosis), hypertrophic or dilated cardiomyopathies, and thyrotoxicosis.
II. ANGINA
A. History should ascertain whether risk factors (DM, smoking, HTN, family history of early coronary disease) exist. Pain is characterized by character, location, duration, radiation, and exacerbating (emotional stress, eating, cold weather) or alleviating (rest, medications) factors. Pain is often a “pressure” described as “heavy” or “grip-like” and may radiate to the arm or jaw. Women, elderly, and diabetic patients may present with atypical symptoms including nausea, vomiting, mid-epigastric pain, or sharp chest pain. An unstable, escalating constellation of symptoms must be differentiated from a stable anginal pattern. Severe angina of recent onset, angina at rest, or angina of increasing duration, frequency, or intensity is classified as unstable and considered a part of the spectrum of ACS.
B. Physical Examination is often nonspecific. Findings of distress, anxiety, tachycardia, HTN, an S4 gallop, pulmonary rales, xanthomas, or peripheral atherosclerosis may be evident. Pulmonary edema, a new or worsening murmur of mitral regurgitation, and angina with hypotension are associated with a high risk of progression to nonfatal MI or death.
C. Noninvasive Studies
1. The sensitivity of resting ECG abnormalities for coronary heart disease events is low. The prevalence of the most common ECG abnormalities (Q waves, left ventricular hypertrophy, bundle-branch blocks, and ST-segment depression) ranges from 1% to 10%. ECG changes indicative of myocardial ischemia that may progress to MI include new ST-segment elevations at the J-point in two or more contiguous leads that are ≥0.2 mV in leads V2–V3 and ≥0.1 mV in all other leads. Ischemia from coronary vasospasm may present with ST-segment elevation. ST-segment depression or T-wave inversions may also signal risk for MI. Isolated J-point elevation or early repolarization may occur as a normal variant in young, healthy adults. Significant Q waves are suggestive of a prior MI. A bundle branch block (BBB) or artificial pacer may complicate detection of ST-segment or T-wave abnormalities. Serial ECGs in 15-to-20-minute intervals can determine whether ischemia is evolving. In general, reversibility of ECG changes after therapeutic interventions is highly suggestive of ischemia. Table 15.1 outlines the ECG changes associated with specific regions of ischemic myocardium.
2. Exercise ECG. Exercise stress testing involves monitoring HR, blood pressure (BP), and ECG while the patient performs a standardized exercise protocol on a treadmill or bicycle. Indications for exercise testing include presumptive obstructive CAD, risk assessment and prognosis in suspected or known CAD, the presence of multiple risk factors in asymptomatic patients, and certain postrevascularization situations. Each level of exercise reflects increased O2 uptake or metabolic equivalents (METs). A single MET is 3.5 cm3 O2/kg/min. Most protocols initiate exercise at 3 to 5 METs and increase by several METs every 2 to 3 minutes. The test is discontinued for moderate to severe angina, significant arrhythmias, exhaustion, CNS symptoms, decrease in BP >10 mmHg from baseline, severe hypertensive response (SBP >250 mmHg or DBP >115 mmHg), significant ECG changes, or evidence of poor peripheral perfusion. In general, achievement of a predicted maximal HR of 85% or a double product (HR × SBP) of 20,000 is necessary for a diagnostic negative test. A positive test (i.e., one which demonstrates ST-segment elevation or depression, a fall in BP with exercise, development of serious arrhythmias, or the development of anginal chest pain with exercise) indicates a high likelihood of significant CAD. Sensitivity for obstructive CAD increases with the severity of stenotic disease. ECG changes occurring at lower workloads are generally more significant than those at higher workloads. Positive tests should prompt consideration of cardiac catheterization and revascularization. Absolute contraindications to exercise stress testing include a recent MI (within 2 days), high-risk unstable angina, symptomatic severe aortic stenosis, symptomatic heart failure, arrhythmia causing hemodynamic compromise, acute pulmonary embolus, aortic dissection, inability to exercise, and an uninterpretable ECG. Relative contraindications include uncontrolled HTN (SBP >200 mmHg or DBP >110 mmHg), moderate stenotic valvular disease, tachy- or bradyarrhythmias, hypertrophic cardiomyopathy, left main coronary stenosis, high-grade atrioventricular block, and a physical or mental limitation to exercise.
| Location of Ischemia or Infarct by Electrocardiographic Criteria | |
Region | Leads | Coronary Artery |
Anteroseptal | V1–V2 | Left anterior descending |
Anterior | V2–V5 | Left anterior descending |
Apical | V5–V6 | Left anterior descending |
Lateral | I, aVL | Circumflex |
Inferior | II, III, aVF | Right coronary artery |
Posterior (Inferolateral)a | Large R wave in V1, V2, or V3 with ST depression | Right coronary artery |
Right ventricular | V3R, V4R | Right coronary artery |
aThe posterior and inferolateral designate the same segment of the left ventricle. A posterior myocardial infarction may be the result of compromise of the right coronary artery or an obtuse marginal branch of the circumflex artery.
3. Myocardial injury enzymes. Biomarker analysis is undertaken immediately to help determine whether angina is stable or unstable. Negative results within 6 hours of symptom onset necessitate further testing at 6-hour intervals if ACS is suspected. When damaged (e.g., by trauma or infarction), cardiac myocytes release various proteins such as creatine kinase-MB fraction (CK-MB), troponin, and myoglobin into the blood. Myocardial troponin is the most accurate marker. Troponin is substantially more sensitive than CK-MB related to the fact that more troponin is found in the heart per gram of myocardium and a greater percentage released from tissue arrives in the blood stream. Troponin is highly specific for cardiac injury, and rare false positives are inherent to any immunoassay testing. In the setting of renal failure, troponin levels may be chronically elevated, and the trend from baseline is key to diagnosing acute injury. Other causes of troponin elevation include advanced heart failure, subarachnoid hemorrhage, acute pulmonary embolism, acute critical illness, acute pericarditis, direct trauma, and myocarditis. Troponin levels may be especially valuable in the perioperative period where CK-MB may be elevated for other reasons and may not reflect myocardial necrosis.
4. Radionuclide perfusion imaging assesses both myocardial perfusion and function in a patient with stable angina.
a. Exercise myocardial perfusion imaging. Thallium-201 is a radioactive potassium analog that is avidly extracted by viable myocardium in proportion to regional myocardial blood flow during exercise. Regions of decreased uptake correlate with the severity of coronary stenosis supplying the regions. Imaging after rest may show a “fixed defect” that takes up no tracer at all, which represents an area of prior infarct, while a “reversible defect,” a region that regains uptake, is considered to be myocardium at risk of ischemia.
b. Exercise radionuclide ventriculography. Intravenous technetium-99m sestamibi accumulates in myocardium in proportion to myocardial blood flow, and multiple ventricular images that are synchronized to the cardiac cycle are acquired at rest and during stress. Ischemia is suggested by regional wall motion abnormalities and the inability to increase left ventricular ejection fraction (LVEF) during exercise.
c. Pharmacologic stress perfusion imaging. Patients not able to undergo exercise testing due to inability to perform moderate physical activity or disabling comorbidity can undergo pharmacological stress imaging. Adenosine and dipyridamole are coronary vasodilators commonly used in stress imaging. Adenosine increases blood flow in disease-free coronary vessels. Dipyridamole inhibits cellular uptake and degradation of adenosine, indirectly increasing coronary flow in disease-free vessels. Since stenotic areas are already maximally dilated, the dilation of disease-free vessels creates differential flow patterns upon coronary imaging. Both drugs may cause angina, headache, or bronchospasm, and must be used with caution in patients with obstructive pulmonary disease. The inotrope dobutamine increases HR, SBP, and contractility, causing increased blood flow secondarily. Imaging after dobutamine administration may show heterogeneous flow due to nondilating stenotic areas.
D. Invasive Studies. Coronary angiography remains the gold standard for quantifying the extent of CAD and guiding percutaneous coronary intervention (PCI; e.g., angioplasty, stenting, atherectomy) or coronary artery bypass grafting (CABG). Coronary angiography will reveal cardiac and coronary anatomy, wall motion abnormalities, and hemodynamic parameters (e.g., ejection fraction). A coronary obstruction is clinically significant when more than 70% of the luminal diameter is narrowed or when more than 50% of the luminal diameter of the left main coronary artery is narrowed. Coronary angiography is not without risk; the mortality rate is approximately 0.5% to 1%. Complications associated with PCI include coronary dissection or perforation, coronary intramural hematoma, distal embolization, myocardial ischemia and infarction, access site bleeding or hematoma, retroperitoneal hematoma, acute kidney injury, stroke, thrombocytopenia, arrhythmias, infection, and effects of radiation exposure.
E. Medical Management. Once it has been determined that angina is stable, appropriate management should be initiated.
1. Smoking cessation
2. BP control to a goal of <140/90 mmHg
3. Dietary modification
4. Medically supervised physical activity and weight reduction
5. Aspirin (ASA) acts by irreversibly inhibiting cyclooxygenase-1 (COX-1) in platelets thereby preventing formation of thromboxane A2 and decreasing platelet aggregation. ASA should be started at 81 to 325 mg daily if no contraindications exist (e.g., allergic reaction, bleeding disorder, coagulopathy).
6. Angiotensin-converting enzyme (ACE) inhibitors reduce sympathetic tone, control HTN, and have a survival benefit in heart failure (HF). In the absence of contraindications (e.g., angioedema, renal artery stenosis), ACE-inhibitors should be started in all patients with an ejection fraction less than 40%, especially if HTN, DM, or renal disease coexists. The benefit of ACE-inhibitors in patients with stable CAD without HF is uncertain in the literature.
7. β-Blockers should be initiated in all patients with a history of MI or ACS unless contraindicated (Table 15.2).
8. HMG-CoA reductase inhibitors (“statins”) improve lipid profiles, limiting progression of atherosclerotic disease and coronary calcium deposition while stabilizing existing plaques. Statin therapy lowers the risk of coronary heart disease death, recurrent MI, stroke, and the need for revascularization. Contraindications include allergic reaction and active liver disease.
| Contraindications to β-blockers in ACS | |
• Marked first-degree AV block
• Any form of second- or third-degree AV block
• Active asthma or history of reactive airway disease
• Evidence of left ventricular dysfunction, heart failure, or low output state
• High risk for shock (e.g., time delay to presentation, lower blood pressure)
F. Invasive Management for stable angina consists of PCI or CABG and depends upon the patient’s response to medical management as well as the underlying coronary artery disease found on coronary angiography (see Section II.B.)
III. ACUTE CORONARY SYNDROMES. Regardless of whether ACS is due to unstable angina (UA), NSTEMI, or STEMI, the goal is to minimize ischemic time and, when appropriate, initiate reperfusion therapy with thrombolysis, PCI, or CABG.
A. The history should attempt to differentiate UA from an acute MI. Symptoms are often indistinguishable (see Section II.A).
B. The physical examination is unlikely to distinguish UA from an acute MI (see Section II.B).
C. As for angina, noninvasive studies include the ECG and cardiac enzymes.
1. A 12-lead ECG should be obtained as soon as possible to determine whether a STEMI is occurring and immediate revascularization is needed. Transient ST-segment elevations (>0.05 mV) that resolve with rest likely indicates true ischemia and severe underlying CAD. ST-segment elevation <0.05 mV, ST-segment depression, or T-wave inversion is more often associated with NSTEMI or UA. A normal ECG does not rule out MI, as up to 6% of patients later confirmed to have had MIs may present as such. Serial ECGs are more accurate than an isolated study.
2. The best biomarker confirmation is a rise in cardiac troponin I or T (>99th percentile). If clinical suspicion for MI is high, cardiac troponin should be drawn at 0, 6 to 9, and 12 to 24 hours. Cardiac troponin I and T are highly specific for myocardial damage but do not indicate the mechanism of damage. CK-MB is less sensitive, but may be substituted if troponin measurement is not available (see Section II.C.3).
3. The chest x-ray (CXR) can detect MI complications such as pulmonary venous congestion and can help to rule out aortic dissection, pneumonia, pleural effusion, and pneumothorax. Pulmonary venous congestion and increased cardiothoracic ratio (>50%) found on initial CXR is a marker for increased mortality in acute MI.
4. Transthoracic echocardiography (TTE) can help establish the diagnosis, prognosis, location, extent, and complications related to acute MI. Findings may include regional wall motion abnormalities, ventricular septal defect (VSD), papillary muscle rupture, free wall rupture, valvular pathology, thrombus formation, and changes in global ventricular function. If TTE is available and the echocardiographer is experienced, this modality offers a convenient method by which a more complete clinical picture can be obtained.
D. Management of Acute MI
1. The general approach to acute MI focuses on minimizing total ischemic time (onset of symptoms to start of reperfusion). In a PCI-capable hospital, primary PCI should be accomplished within 90 minutes of STEMI. Initiation of thrombolysis within 30 minutes is the system goal for STEMI when PCI is not available. Early invasive strategy (diagnostic angiography, PCI) is indicated in UA/NSTEMI in the setting of refractory angina or hemodynamic and/or electrical instability. Patients initially stabilized on medical therapy with UA/NSTEMI for whom an initial invasive (PCI) strategy is chosen, early angiography and intervention within 24 hours reduces ischemic complications.
2. Supportive measures. Supplemental O2, IV access, routine vitals, and continuous ECG should be initiated. Few data exist to support or refute the value of routine O2 in ACS. Supplemental O2 is indicated for patients with HF or hypoxemia (SpO2 <90%). Patients with severe HF or cardiogenic shock may need either noninvasive positive pressure ventilation or intubation and mechanical ventilation. Laboratory studies include electrolytes with magnesium, lipid profile, and complete blood count to detect anemia. Continuous pulse oximetry is critical to evaluate oxygenation, especially in patients with HF or cardiogenic shock.
3. Treatments. Unless contraindicated, a β-blocker, ASA, anticoagulation, ACE-inhibitor, statin, and possibly a thienopyridine (e.g., clopidogrel) should be administered.
a. β-BLOCKADE appears to reduce myocardial O2 consumption by slowing the HR and reducing cardiac contractility. However, there is evidence that β-blockers may be detrimental in patients with acute HF (Table 15.2). If no contraindications exist, β-blockers should be initiated in the first 24 hours. Initially, metoprolol 5 mg IV every 5 minutes up to a total of 15 mg may be given, titrating to HR and BP. If tolerated, metoprolol 25 to 50 mg orally every 6 hours can be administered for 2 days and then increased up to 100 mg orally twice daily as tolerated. Carvedilol 6.25 mg administered orally twice a day, titrated up to a maximum of 25 mg twice a day, may reduce mortality in patients with an acute MI and LV dysfunction.
b. The benefit of calcium-channel blockers (CCBs) in ACS is related to symptom reduction in patients already receiving β-blockers and nitrates or who cannot tolerate these agents. There is no beneficial effect on infarct size or the rate of reinfarction when CCBs are initiated during the acute phase of STEMI. Nifedipine 30 to 90 mg orally can be administered. If tolerated, a longer-acting agent such as Diltiazem (slow release) 120 to 360 mg once daily or Verapamil (slow release) 120 to 480 mg by mouth once a day may be initiated.
c. Nitrates increase venous capacitance, thus decreasing preload and cardiac work. Nitrates dilate epicardial coronary arteries and collateral circulation, and also inhibit platelet function. Evidence does not support routine long-term nitrate therapy in MI unless pain persists. IV nitroglycerin (NTG) is started at 10 μg/min and titrated to symptom relief or BP response. Patients with acute MI or HF, large anterior infarcts, persistent ischemia, or HTN may benefit from IV NTG for the first 24 to 48 hours. Those with continuing pulmonary edema, recurrent ischemia, or recurrent angina may benefit from even more prolonged use of NTG. Ideally, IV NTG should be changed to an oral route within 24 hours; for instance, isosorbide dinitrate 5 to 80 mg orally 2 to 3 times per day. NTG is generally contraindicated in patients with hypotension, marked bradycardia or tachycardia, RV infarction, or 5’phosphodiesterase inhibitor use within 24 to 48 hours.
d. ACE-inhibitors should be initiated within 24 hours to all patients with anterior MI, HF, or ejection fraction ≤40% unless contraindicated. An angiotensin II receptor blocker (ARB) can be substituted in patients intolerant of ACE-inhibitors. ACE-inhibitors reduce fatal and nonfatal major cardiovascular events in patients with STEMI independent of the use of other pharmacotherapies (e.g., ASA, β-blocker). Lisinopril 2.5 to 5 mg per day can be administered initially with higher titration as tolerated. Caution should be taken in patients with renal failure, hyperkalemia, or hypotension.
e. HMG-CoA reductase inhibitors (“statins”) should be initiated in all patients with ACS without contraindications. Statin therapy lowers the risk of coronary heart disease death, recurrent MI, stroke, and the need for revascularization. Atorvastatin 80 mg daily has been shown to reduce death and ischemic events in patients with ACS.
f. Analgesia. Morphine sulfate (1–5 mg IV) for analgesia and anxiolysis is reasonable unless contraindicated by hypotension or history of morphine intolerance. Associated modest reductions in HR and BP decrease O2 consumption. Side effects include hypotension, respiratory depression, bradycardia, and nausea. Except for ASA, nonsteroidal anti-inflammatory drugs (NSAIDs) and selective cyclooxygenase II enzyme (COX-2) inhibitors may be associated with an increased risk of death, reinfarction, cardiac rupture, HTN, renal insufficiency, and HF. NSAIDs and COX-2 inhibitors are contraindicated in patients with STEMI and should be discontinued in patients taking them prior to hospitalization.
g. Antiplatelet therapy should be considered in all patients presenting with ACS. Agents are chosen on the basis of whether the proposed management will be conservative or invasive (e.g., CABG or PCI, which yield an increased risk of hemorrhage). A combination of ASA and clopidogrel has been shown to reduce recurrent coronary events in the posthospitalized ACS population. For UA/NSTEMI patients treated medically without stenting, ASA should be prescribed indefinitely and clopidogrel should be prescribed for up to 12 months in the absence of contraindications. For ACS patients treated with a bare metal stent (BMS) or drug-eluting stent (DES), ASA should be prescribed indefinitely and clopidogrel should be prescribed for at least 12 months (continuation may be considered past 12 months). For patients receiving DES for a non-ACS indication, clopidogrel should be given for at least 12 months and continuation may be considered past 12 months. For patients receiving BMS for a non-ACS indication, clopidogrel should be given at least 1 month and ideally up to 12 months. If bleeding risks outweigh the benefits of platelet inhibitors, earlier discontinuation should be considered.
1. ASA 325 mg by mouth should be given to all patients, unless contraindicated by hypersensitivity or a history of major GI bleeding. ASA acts by irreversibly inhibiting cyclooxygenase-1 (COX-1) in platelets thereby preventing formation of thromboxane A2 and decreasing platelet aggregation.
2. If a contraindication to using ASA exists, a 300-mg oral loading dose of clopidogrel followed by 75 mg once a day can be started. Clopidogrel and ASA are given together if PCI management is planned.
3. Glycoprotein IIb/IIIa inhibitors may be added as a third agent as part of a triple antiplatelet therapy in select high-risk patients. The risk of using these agents as a part of triple antiplatelet therapy may outweigh their potential benefit when there is concern for increased bleeding risk or in non–high-risk patients.
h. Further anticoagulation depends upon whether conservative or invasive management is pursued. Unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), a direct thrombin inhibitor (e.g., bivalirudin), or a factor Xa inhibitor (e.g., fondaparinux) may be chosen.
1. UFH has the advantage of common use but the disadvantage of a risk of heparin-induced thrombocytopenia (HIT). Initial dosing should be titrated to target an aPTT of 1.5 to 2 × normal. A normal dose is a 60-unit-per-kilogram bolus followed by a 12-unit-per-kilogram-per-hour infusion.
2. LMWH has a lower risk of HIT and, being given subcutaneously, it is generally thought to be easier to administer. Concerns about the ability to monitor the effectiveness of LMWH compared with UFH, as well as a concern that reversal with protamine may be difficult in the setting of planned PCI, have limited its use.
3. Direct thrombin inhibitors have no risk of HIT but are associated with more bleeding complications and an inability to reverse effects with either protamine or FFP. Bivalirudin is a direct-acting synthetic antithrombin that acts against clot-bound thrombin with a very short half-life (25 minutes). Bivalirudin can be useful in patients with HIT.
4. Fondaparinux, a factor Xa inhibitor, is efficacious in anticoagulation management after ACS and confers a lower risk of bleeding when compared with UFH and GP IIb/IIIa inhibitors. The lack of antidote and catheter thrombosis during PCI limit its use.
i. Magnesium dilates coronary arteries, inhibits platelet activity, suppresses automaticity, and may protect against reperfusion injury. Prophylactic administration in acute MI does not improve mortality but may reduce arrhythmias at the cost of an increased incidence of hypotension, bradycardia, and flushing. Current evidence does not support magnesium as an effective treatment in ACS. However, supplemental magnesium is indicated for hypomagnesemia and torsades de pointes. Hypomagnesemia is corrected with magnesium sulfate 2 g IV over 30 to 60 minutes, while torsades de pointes is treated with 1 to 2 g IV over 5 minutes. Since most magnesium in the body is intracellular, hypomagnesemic patients may require multiple replacement doses to achieve normal levels.
j. Insulin should be administered to patients presenting with ACS and hyperglycemia to maintain blood glucose levels <180 mg/dL while avoiding hypoglycemia.
k. Pharmacologic or mechanical reperfusion therapy reduces infarct size and mortality and improves function, even after a prolonged period. Transient myocardial impairment (“stunned myocardium”) may exist after the injury is reversed. In general, PCI is preferred for STEMI with a goal to be performed within 90 minutes of initial presentation to a PCI-capable hospital. Immediate transfer to a PCI-capable hospital is recommended if initial presentation is at a non-PCI-capable hospital with the goal of first medical contact to reperfusion time of <120 minutes. If the anticipated time to PCI is >120 minutes, thrombolysis is preferred in the absence of contraindications.
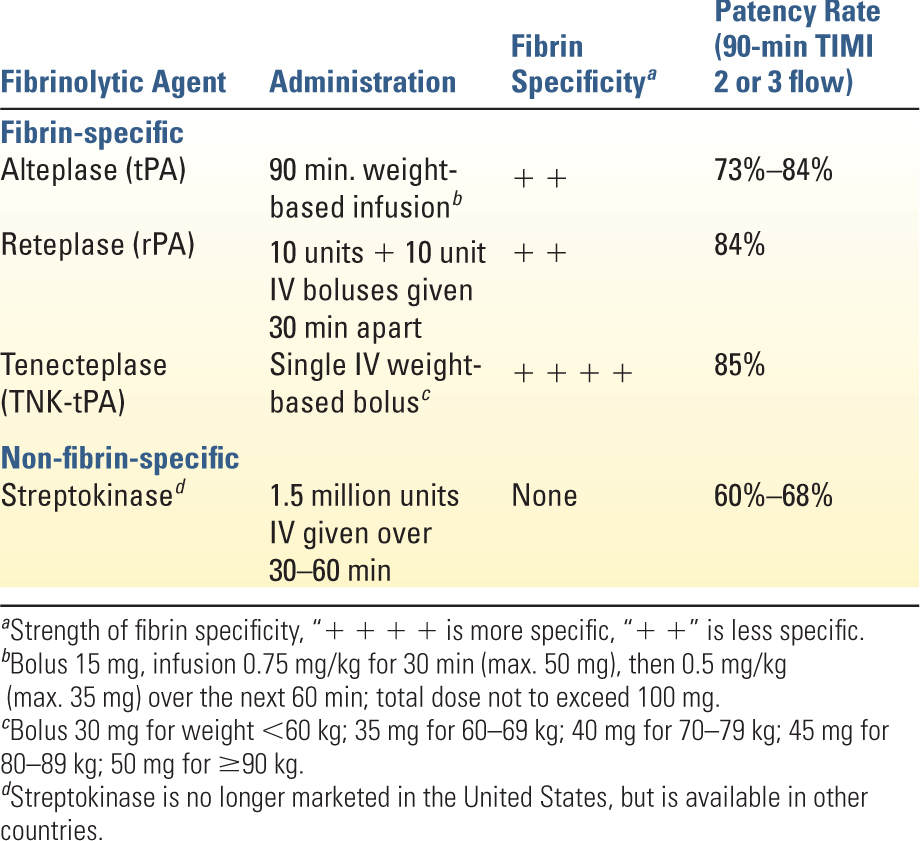
1. Thrombolysis is only indicated in the presence of ST-segment elevation >0.1 mV in at least two contiguous leads when PCI cannot be performed within 120 minutes of first medical contact and no contraindications exist. Thrombolysis yields the greatest benefit when initiated within 6 hours of symptom onset, although definite benefit exists even at 12 hours. Patients presenting within 12 to 24 hours but with continuing symptoms may also benefit. Response to therapy results in improvement in ST-segment elevation and resolution of chest discomfort. Persistent symptoms and ST-segment elevation 60 to 90 minutes after thrombolysis are indications for urgent angiography and possible PCI. Thrombolysis offers no benefit in patients without ST-segment elevation or in those with MI complicated by HF or cardiogenic shock. Table 15.3 shows a comparison of the commonly used thrombolytic agents. Side effects are bleeding, hypotension, and allergic-type reactions. Absolute contraindications are any prior intracranial hemorrhage, cerebral vascular malformation or malignant intracranial neoplasm, suspected aortic dissection, active bleeding, intracranial or intraspinal surgery within the past 2 months, significant head trauma or ischemic stroke within the past 3 months (does not include an acute CVA within 4.5 hours), or severe uncontrolled HTN unresponsive to medical therapy. Relative contraindications include chronic, severe, poorly controlled HTN, significant HTN on presentation (SBP >180 mmHg or DBP >110 mmHg), history of prior ischemic stroke more than 3 months ago, dementia, known intracranial pathology not covered in absolute contraindications, traumatic or prolonged cardiopulmonary resuscitation (>10 minutes), major surgery within the past 3 weeks, internal bleeding within the past 2 to 4 weeks, concurrent anticoagulation, noncompressible vascular puncture sites, pregnancy, or active peptic ulcer disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree