I. INTRODUCTION
Asthma and COPD are common clinical conditions that are characterized by airflow obstruction measured with spirometry. Obstruction is usually defined by a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of <0.7 or the lower limit of normal based on a 95% confidence interval in population-based studies of healthy subjects of similar sex, height, age, and race. Asthma and COPD can lead to respiratory failure and ICU admission during acute exacerbations of these conditions, or they can complicate the ICU care of a patient who is admitted for other reasons or who is admitted from the operating room. Since the effect of each condition on lung physiology is very similar and they share many treatments, they are discussed together here.
A. Definitions
1. COPD—GOLD definition
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines COPD as “a common preventable and treatable disease, characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases. Exacerbations and comorbidities contribute to the overall severity in individual patients.”
2. Asthma—GINA definition
The Global Initiative for Asthma (GINA) defines asthma as “a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment.”
3. Status asthmaticus
Acute severe asthma, formerly known as status asthmaticus, is defined as severe asthma unresponsive to repeated courses of β-agonist therapy such as inhaled albuterol, levalbuterol, or subcutaneous epinephrine. We will use both terms interchangeably. Status asthmaticus is not a distinct condition, but rather the most severe manifestation of an asthma exacerbation along a continuous spectrum of severity, thus the preferred term acute severe asthma. These patients often require ventilatory assistance in addition to inhaled and intravenous medication. Treatments for acute severe asthma and all asthma exacerbations in general will be discussed together as they are essentially the same, except that more treatments are added as the patient’s condition worsens.
B. Epidemiology
1. COPD
a. Recent epidemiological studies report a prevalence of COPD in developed countries of 8% to 10% in adults older than 40 years, with 1.4% having severe COPD. While the prevalence of COPD has been stable in the United States in recent years, it is increasing in women and decreasing in men, as is mortality attributable to COPD. Acute exacerbation of COPD accounts for approximately 2.5 hospitalizations per 10,000 people per year and has an in-hospital mortality of approximately 5%. Currently, COPD is the third cause of death in the United States, where it affects more than 15 million people.
b. A recent large study reported that approximately 9% of patients admitted to the ICU have a diagnosis of COPD and roughly one-third of them, corresponding to 2.5% of all admissions, are due to acute respiratory failure from COPD exacerbation.
c. COPD is an independent risk factor for ICU mortality even when it is a comorbid condition rather than the primary cause for admission.
2. Asthma
a. Asthma affects more than 25 million people in the United States and accounts for approximately 1.25 million hospitalizations per year, corresponding to an annual hospitalization rate of 13.5 per 10,000 individuals.
b. About 10% of asthma hospitalizations result in admission to the ICU and approximately 2% require endotracheal intubation.
c. Both ICU admission and intubation, as well as the presence of comorbidities, increase the odds of in-hospital death. In-hospital asthma mortality is approximately 0.5%.
C. Physiology
1. Airway inflammation
Although both asthma and COPD are characterized by airway inflammation, there are important differences in the cause of the inflammation and the inflammatory cell composition in each condition. Asthma is most commonly caused by an allergic reaction to one or more airborne environmental allergens, whereas COPD is the result of chronic inhalation of smoke. The airway inflammation in asthma is dominated by eosinophils, whereas the inflammation in COPD is largely composed of neutrophils. In fact, in atopic asthma, uptake of [18F] fluorodeoxyglucose measured with positron emission tomography in inflamed lung regions, an index of inflammatory cell activation, correlates with eosinophil counts from those regions. In contrast, in COPD, pulmonary [18F] fluorodeoxyglucose uptake is related to neutrophil infiltration. Another major distinction between the two is that while both can have airway wall inflammation, edema, and constriction, COPD very often results in loss of lung tissue, termed emphysema. Thus, for COPD, there has historically been a distinction made between those patients who have primarily airway disease from those who have primarily emphysema, though there is great overlap between the two phenotypes. In fact, it is accepted that there are likely many different phenotypes, both in asthma and COPD, and recognition of these phenotypes may lead to important treatment decisions.
2. Phenotypes
There is a growing appreciation that patients with asthma and COPD are very heterogeneous and likely have multiple causes for their intermittent airway obstruction. In asthma, at least five clinical phenotypes have been identified: (1) early-onset allergic, (2) late-onset eosinophilic, (3) exercise induced, (4) obesity related, and (5) neutrophilic. COPD also represents a heterogeneous syndrome with chronic bronchitis and emphysema being recognized early on as distinct phenotypes. Chronic bronchitis has classically been associated with cough, obesity, hypoxemia, and hypoventilation, whereas emphysema has been associated with muscle wasting and hyperinflation but preserved gas exchange. COPD can be further characterized by other factors, such as the degree, type, and distribution of emphysema, or nonspirometric physiologic parameters, such as diffusing capacity and hyperinflation. As with asthma, classification schemes of COPD phenotypes based on frequency of exacerbations, radiologic parameters, and inflammatory biomarkers are being developed. It is important to note that there is an “overlap” phenotype that is characterized by a positive bronchodilator response, sputum eosinophilia, a personal history of asthma before age 40, high total IgE level, and a history of atopy. These patients seem particularly prone to frequent exacerbations and lung function decline.
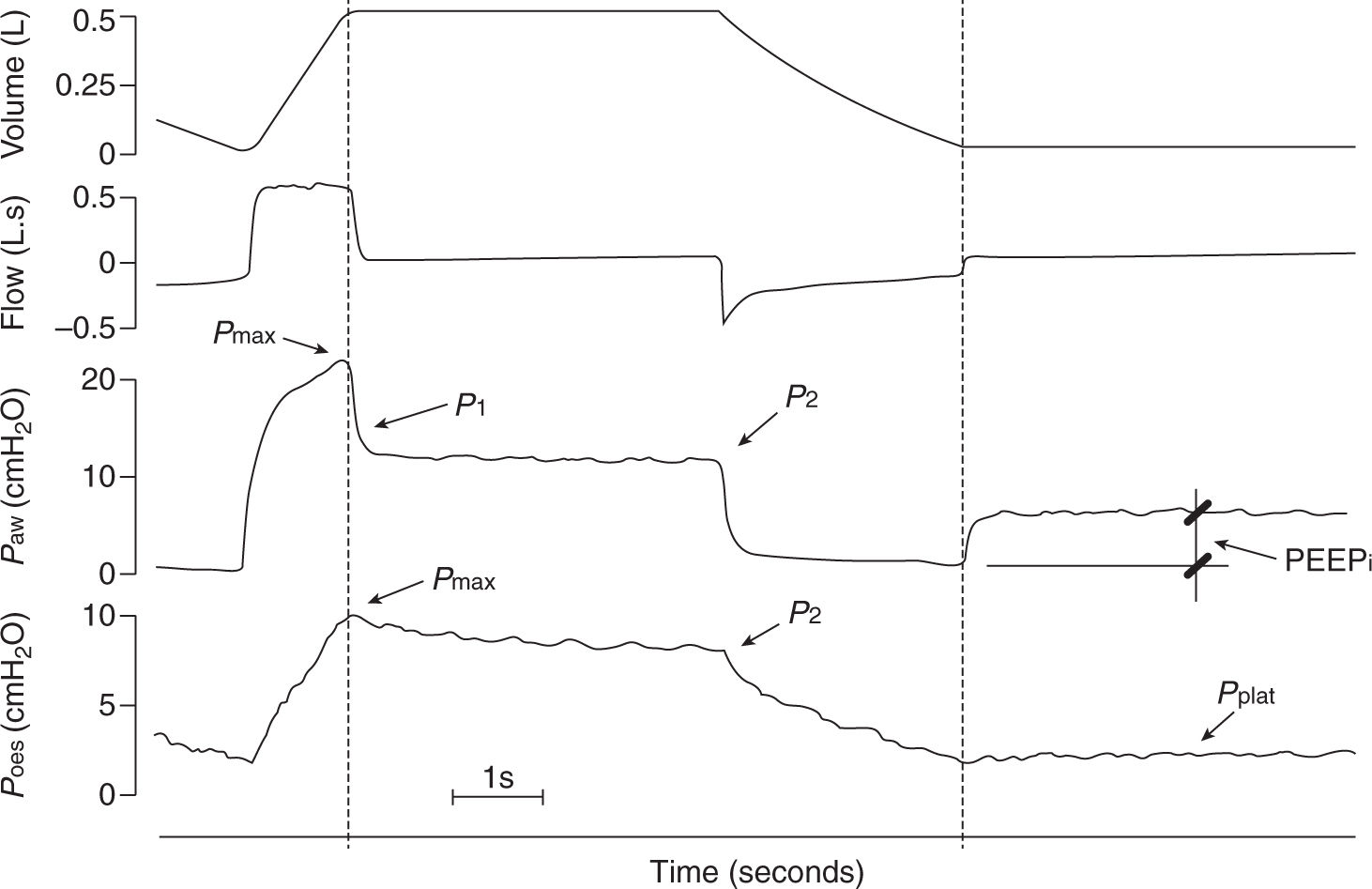
FIGURE 20.1 Tracings of volume (obtained by integration of the flow signal), flow, airway pressure (Paw) and esophageal pressure (Poes) in a representative patient with chronic obstructive pulmonary disease (COPD) during an end inspiratory occlusion followed by an end expiratory occlusion. Dashed lines indicate timing of occlusions. Pmax, maximum value; P1, zero flow value; P2, plateau value; PEEPi, intrinsic positive end-expiratory pressure is the static end-expiratory recoil pressure of the respiratory system; Pplat, plateau value of Poes after end-expiratory occlusion. Values of Paw, Pmax, used for calculations were corrected for the resistive pressure drop due to the artificial airway. (From Musch G, Foti G, Cereda M, et al. Lung and chest wall mechanics in normal anaesthetized subjects and in patients with COPD at different PEEP levels. Eur Respir J 1997;10:2545–2552, with permission.)
3. Airflow obstruction
a. The pathophysiologic hallmark of both asthma and COPD is airway narrowing with ensuing increase in respiratory resistance and wheezing.
b. In asthma, airway narrowing is due predominantly to contraction of smooth muscle cells in the airway wall, airway wall edema, and mucus plugging. Consequently, airway narrowing is present during both inspiration and expiration. The narrowed airways retard expiratory flow, resulting in dynamic hyperinflation, that is, an increase in end-expiratory lung volume above functional residual capacity due to lack of equilibration between alveolar pressure and pressure at the airway opening. In fact, bronchoconstriction can be so severe that airways completely occlude, trapping gas behind them. Interestingly, the size of these gas-trapping areas termed ventilation defects appears to be less in the prone than in the supine position, suggesting a rationale for the use of prone positioning in status asthmaticus.
c. In COPD with emphysema, airway narrowing is due predominantly to intrapulmonary airway collapse during expiration. Because of the destruction of lung tissue, the tethering forces exerted by the parenchymal septae on the intrapulmonary airway wall are decreased. Consequently, during expiration, when the transmural pressure in these airways becomes negative (i.e., when the intraluminal airway pressure becomes lower than the surrounding extraluminal airway pressure), the airways collapse and interrupt flow. As flow is interrupted, upstream pressure rises and the airways reopen. As flow restarts, the transmural pressure becomes negative and flow again stops. This “flutter” pattern is responsible for the phenomenon of expiratory airflow limitation.
d. Expiratory airflow limitation is defined as a state in which expiratory flow is independent of transpulmonary pressure and hence is maximal. In the presence of flow limitation, an increase in upstream pressure, for example, by activation of expiratory muscles, will not result in a higher expiratory flow. The point in the airway tree in which the transmural airway pressure turns negative is called equal pressure point. If this point localizes within the collapsible intrapulmonary airway, it will become a “choke point” that impedes further increases in expiratory flow. Equally ineffective in increasing flow will be a negative pressure applied at the opening of the airway during expiration.
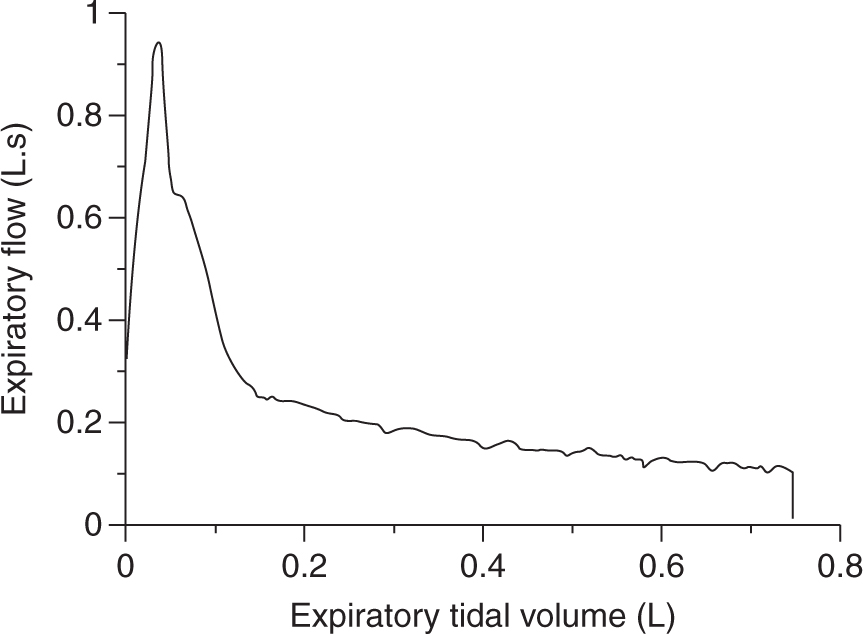
FIGURE 20.2 Expiratory flow volume curve during passive exhalation at zero end-expiratory pressure in a patient with chronic obstructive pulmonary disease. Notice the bowing toward the volume axis (i.e., upward concavity) especially during the first part of exhalation, which suggests the presence of flow limitation. (From Musch G, Foti G, Cereda M, et al. Lung and chest wall mechanics in normal anaesthetized subjects and in patients with COPD at different PEEP levels. Eur Respir J 1997;10:2545–2552, with permission.)
e. Similarly, if pressure at the airway opening is raised, flow will not decrease until the applied pressure is sufficiently high to overcome flutter at the choke point, that is, to render transmural pressure positive along the entire collapsible airway. Levels of applied pressure higher than this critical threshold will decrease expiratory flow, causing further hyperinflation.
f. Consequently, in the presence of expiratory flow limitation, flow is maximal because neither an increase in upstream pressure nor a decrease in downstream pressure will result in higher flows, and an increase in downstream pressure will either not affect flow (“waterfall” effect) or decrease it if the increase is sufficiently high to overcome the critical threshold. In the presence of flow limitation, expiratory flow is thus independent of the pressure gradient along the airway and depends only on lung volume, being higher at higher volumes. However, while it is maximal, the absolute flow is quite minuscule, and prolonged expiratory times would be needed to reach functional residual capacity.
4. Risk factors for death in ICU
For patients admitted to the ICU with respiratory failure from an asthma or COPD exacerbation, the major risk factors for death are need for mechanical ventilation, hyperinflation and auto-PEEP causing hypotension (from decreased venous return), profound acidemia, and barotrauma.
II. ASSESSMENT
A. History
1. A history of poorly controlled asthma as evidenced by nocturnal awakening for asthma symptoms, increased dyspnea and wheezing, increased β-agonist use, increased peak flow variability (>20%), recent or frequent emergency room visits or hospitalizations and a previous history of intubation and mechanical ventilation, and glucocorticoid nonadherence are risk factors for fatal asthma. A history of current smoking also identifies high-risk patients with asthma. Patients with nonallergic asthma, asthma triggered by aspirin, or exercise-induced asthma or patients with asthma who use cocaine or heroin have a higher risk of mortality, and these factors should be assessed. A history of rapid improvement with intubation can often be a clue to an upper airway cause of respiratory failure, and in particular a condition termed paroxysmal vocal cord motion. Patients with this condition often have mild asthma and a history of frequent severe attacks, many times with intubation followed by rapid extubation.
2. For patients with COPD, risk factors for mortality include multiple admissions for exacerbations, intubation and mechanical ventilation, age (both younger and older age groups), lower BMI, history of lung cancer, and cardiovascular comorbidity. For COPD patients, it is important to establish the patient’s baseline arterial PaCO2 (or try to infer it from the patient’s baseline bicarbonate level). This serves two purposes—a warning that the patient may be particularly susceptible to the hypercarbic effects of supplemental oxygen (discussed below) and as a target for setting minute ventilation should mechanical ventilation become necessary.
B. Physical Examination
1. Appearance
Patients with severe exacerbations of asthma or COPD are often unable to lie down, so they are often sitting upright or leaning forward slightly. A patient who is unable to speak in full sentences may be nearing respiratory arrest. As with any patient in respiratory failure, it is important to note the patient’s color to assess perfusion and oxygenation. A patient in respiratory failure without a previous diagnosis of COPD is likely to have a maximum laryngeal height (the distance from the sternal notch to the thyroid cartilage) of less than 4 cm at end exhalation. This is caused by hyperinflation of the lungs that pulls the trachea downward.
2. Assess for intubation difficulty
a. While neither COPD nor asthma per se have been associated with increased incidence of difficult intubation, the association of obesity with asthma and early COPD can make the intubation of these patients more challenging than in the general population. In contrast, patients with moderate to severe COPD (GOLD stages 3 and 4) have a lower body mass index.
b. Irrespective of body mass, a thorough assessment of the airway (e.g., Mallampati class, thyromental distance, receding mandible, protruding upper incisors) should be performed in all patients with obstructive pulmonary disease admitted to the ICU even if they do not yet require mechanical ventilation. In fact, these patients can deteriorate rapidly and require emergent intubation when gas exchange is already impaired.
3. Auscultation
Wheezing may be audible even without a stethoscope and should be expiratory and polyphonic, indicating that it is coming from multiple small airways. A monophonic wheeze might indicate a single large airway obstruction. Stridor indicates obstruction that is extrathoracic, usually at the vocal cords or above. In patients with COPD and asthma who appear to be in respiratory distress, lack of wheezing or vesicular (normal) breath sounds can be a sign of impending respiratory arrest as ventilation becomes so poor that very little sound is made with each breath. In COPD patients, it may be difficult to hear breath sounds normally, since they may have very little lung tissue left through which to transmit airway sounds.
4. Accessory muscle use
Patients with exacerbations of asthma and COPD are often anxious, sitting upright with arms extended, supporting the upper chest. This position allows the abdominal contents to pull downward on the diaphragm and expand the lungs. It also allows the rib cage to be expanded and the accessory muscles of the neck and shoulders to pull upward on the rib cage when inhaling. Accessory muscle use and retraction of the rib cage or of the intercostal muscles is an ominous sign and indicates increased mechanical work to ventilate. Even more ominous is “abdominal paradox” where the usual abdominal protrusion during inhalation is reversed, such that the abdomen pulls inward during inspiration. This indicates that the diaphragm is either not functioning (due to paralysis or paresis) or the lungs are so hyperinflated that the diaphragm is at a mechanical disadvantage and can no longer pull the lungs downward. Once this happens, only the accessory muscles are able to lower intrathoracic pressure and this draws the abdominal contents inward toward the thorax. Hyperinflation of the lungs with a flattened diaphragm can also result in “Hoover’s Sign,” where the lower costal margin is drawn inward during contraction of the diaphragm. Although often a sign of respiratory failure in COPD, it can be seen in stable outpatients, but indicates more severe disease.
C. Laboratory
1. Arterial blood gas
a. Depending on the severity of disease, COPD patients may present a chronic respiratory acidosis (i.e., increased arterial PaCO2) that is partially offset by renal tubular excretion of H+ and reabsorption of HCO3–, resulting in a compensatory metabolic alkalosis. CO2 retention is a result of both hypoventilation due to airflow obstruction and ventilation-perfusion mismatch due to heterogeneous parenchymal involvement in COPD. To preserve the equilibrium of the Henderson–Hasselbalch equation, these patients present a largely increased HCO3– concentration and base excess. A typical blood gas in this setting would be as follows: PaCO2 = 60 mmHg, pH = 7.36, HCO3– = 33 mEq/L. PaO2 and SaO2 also depend on severity of disease and on whether the patient is on supplemental oxygen therapy, but are often moderately decreased (PaO2 = 60–80 mmHg and SaO2 = 90%–95%).
b. An acute on chronic exacerbation of COPD often results in further CO2 retention and uncompensated respiratory acidosis, in addition to worsening hypoxemia. The presence of hypercapnia and acidosis despite markedly increased bicarbonate, and a base excess is indicative of acute decompensation in acid–base status of these patients.
c. In asthmatics, blood gases are usually normal at baseline, except in very advanced stages of disease when chronic features of obstructive disease appear. During an acute attack, however, PaO2 may decrease below 60 mmHg (SaO2 <90%) and hypercapnia may also ensue. Importantly, because an asthma attack is accompanied by increased respiratory drive, a PaCO2 only mildly elevated (e.g., >42 mmHg) could signify impending respiratory failure because for that level of drive the expected PaCO2 in the absence of significant bronchoconstriction would be much lower.
2. Complete blood count (CBC)
a. A CBC is not usually required during asthma exacerbations but may be warranted in patients with a fever or purulent sputum. Even in the absence of superimposed bacterial infection, these patients may show neutrophilia due to the stress response and concomitant therapy (corticosteroids and β2-agonists). Furthermore, asthma can cause eosinophilia, which is usually mild to moderate, that is, 500 to 1,500 eosinophils/µL.
b. During an acute exacerbation of COPD, the patient may present neutrophilia if the cause of the exacerbation is an acute bacterial infection. However, as for asthma, such leukocytosis could be simply the result of concurrent stress or therapy.
3. Theophylline level
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








