Chemical Agents and Radiation Exposure
Paul Barach
Ernesto Pretto Jr.
CASE SUMMARY
The New York City police stage a raid on a suspected terrorist chemical laboratory on the lower west side of Manhattan at 07:00 AM. The laboratory is located in a warehouse, which is empty when the police arrive and which the terrorists have boobytrapped. As the police enter, the terrorists trigger an explosive detonation that collapses the building, damaging a subway tunnel running underneath it. In the explosion, 50 people—including police, subway passengers, and others in the area—are killed, and several hundred are injured. A plume of yellow vapor escapes from the building site and travels northeast across Manhattan, northern Brooklyn and Queens, and many people on sidewalks are coughing. During the next hour, Manhattan hospitals are besieged with patients complaining of eye irritation and difficulty breathing. Within about 2 hours, hospitals begin to see hundreds of patients with skin erythema and frank blistering. You are called to the emergency room (ER) where the scenario is one of utter chaos and panic. People are rushing around assisting casualties who are arriving in waves. Nurses and ER doctors are shouting and want you to intubate multiple casualties simultaneously. You immediately realize that you need help, and you quickly summon fellow anesthesiologists and respiratory therapists, and anyone with advanced cardiac life support (ACLS) training capable of managing a compromised airway and performing endotracheal intubation. You assume the responsibility of directing airway management and ventilatory support for multiple casualties in acute respiratory distress.
There are over a dozen casualties in the ER like Mr. David Lee, a 45-year-old, 92-kg, banker, complaining of 6 hours of chest pain, nausea, dizziness, itchy eyes and skin, and difficulty breathing. His respiratory rate is 35 breaths per minute, his pulse is 125 bpm and regular, his blood pressure is 190/110, his eyes are red, and he has copious secretions from his mouth and nose. His speech is coherent, and he is oriented to place and time. He is gasping for air and shouting in muffled tones, “I can’t breathe, I can’t breathe, please help me …” His saturation is 92% on room air, and he is profusely sweating. He refuses to wear an oxygen mask. After examining Mr. Lee and the first few patients with similar symptoms, you direct nursing staff to give patients escalating doses of atropine, starting with 1 mg intravenously over 10 minutes, as well as a shot of oxime 2-pralidoxime chloride (PAM), 1 mg intramuscularly. Despite this regimen, several casualties do not improve and continue to deteriorate, so you decide to intubate them but are limited by available resources and their copious secretions. You request additional airway supplies—including masks, endotracheal tubes, Ambu bags, ventilators, suction catheters, and so on—to be brought to the ER and additional personnel to come to the ER immediately to help “bag” patients. After several successful intubations, you notice that the Ambu bags are quickly clogging up, caused by large bronchial secretions. As you are preparing to attempt to resolve this problem, Mr. Lee desaturates, becomes bradycardic, and goes into cardiac arrest. (Case summary modified with courtesy of Roger McIntosh.)
What Baseline Knowledge Is Relevant?
A great deal of public attention and government effort has been devoted to the potential use of weapons of mass destruction (WMD) by terrorists within the United States and around the world. Chemical and biological weapons, such as nuclear weapons, are categorized as WMD because their release causes a large number of potential victims. A successful attack is devastating. A large scale, chemical attack in a metropolitan area could take thousands of lives. Biological and nuclear weapons are capable of killing hundreds of thousands of people and more. In this chapter, we will review the preparation and response in the event of an attack with chemical or radiologic agents. Regrettably, we live in a world where the use of WMD is emerging as a real threat. Although the events of September 11, 2001 have increased our awareness
concerning terrorism, the threat and existence of attacks with WMD has long been a reality. Several countries have developed and stockpiled chemical, nuclear, and biological agents. The actual use of WMD for terrorism has been witnessed in incidents such as the 1994 and 1995 terrorist attacks in Japan where the chemical nerve agent, sarin, was released, causing numerous deaths and hundreds of casualties, including some health care workers.
concerning terrorism, the threat and existence of attacks with WMD has long been a reality. Several countries have developed and stockpiled chemical, nuclear, and biological agents. The actual use of WMD for terrorism has been witnessed in incidents such as the 1994 and 1995 terrorist attacks in Japan where the chemical nerve agent, sarin, was released, causing numerous deaths and hundreds of casualties, including some health care workers.
Owing to these past events, there is an ever-growing awareness by health care professionals concerning the problems of managing casualties of WMD and toxic substance exposure. Although such events are rare, when they do occur, they can cause mass casualties and rapidly overwhelm the existing medical services. There is also risk of toxic injury to health care responders through contamination from the site and the patients themselves. Planning for hazardous materials (HAZMATs) incidents should take place in conjunction with emergency services. In light of these past events, and the probability that others will occur, health care facilities and staff should be prepared to respond to the sequelae of an attack with WMD because these agents pose a threat not only to patients, but health care workers as well. Additionally, the training of health care providers in using protective gear for treating patients exposed to chemical or biological weapons may also prove useful in situations where exposure was unintentional (industrial accidents) or natural (infectious outbreaks). Simple countermeasures can save many lives but require an appropriate emergency response with the availability of basic decontamination, protective equipment, supplies of antidote, and trained rescue and medical teams that are available without delay. Paramedics and emergency department staff, in most cases, would be the front line in a major chemical incident, but they would soon be overwhelmed, and anesthesiologists would certainly be the next in line because of their ability to respond to the need for life support. In any case scenario of WMD, even a small event, anesthesiologists are very likely to be directly involved in either the operative or critical care of WMD victims.
What Are the Historic Considerations of Chemical Agents?
“The effect of chemical agents are so deadly to the unprepared that we can never afford to neglect the question” General J. Pershing, 1919.1
Despite these prescient words from almost a century ago, the management of patients exposed to chemical weapons is not a popular topic, and most health care professionals are unfamiliar with it. Recent surveys have found very few programs that have dedicated curriculum space in undergraduate or graduate medical education.2
This lack of knowledge is particularly perplexing in view of a seven-decade history of modern chemical warfare and the well publicized use of mustard agents and nerve gases during the Iran-Iraq War in the 1980s.3 Disinterest in this topic may be based on the erroneous belief that: (i) chances of a chemical attack are remote; (ii) if chemical attacks occur, the outcome is disastrous; (iii) defense is impossible; and (iv) the casualty and loss rates will be high regardless of their medical care. In fact, the chances of a chemical attack in the current state of the world seem fairly high when you consider the consistent daily reminder of some degree of terror alert on the television news networks. When a terror attack occurs, if it is properly handled, significant morbidity and mortality can be reduced.
TABLE 68.1 World War I Chemical Weapons Casualties | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In our time, World War I was the first large tactical field where chemical weapons were used (see Table 68.1). German units released 150 tons of chlorine gas from 6,000 cylinders near Ypres, Belgium, on April 15, 1915. Although 800 soldiers died, the psychologic effect on the 15,000 men of the Allied armies was devastating. Owing to their relatively easy manufacture and overall devastating effects, mustard gas and nerve agents (NA) are most likely to be used on a modern battlefield or by terrorists as weapons.
What Are the Clinical Signs and Symptoms of Victims Exposed to Nerve Gas?
▪ CLINICAL EFFECTS
The clinical effects of nerve gas depend on the route and degree of exposure. The initial effects from exposure to vapor are not the same as those from a liquid droplet on the skin. A small concentration (30 mL) of vapor affects the eyes, the nose, and the airways to produce miosis, rhinorrhea, bronchorrhea, and bronchoconstriction. A small, sublethal droplet on the skin will cause localized sweating and less commonly localized fasciculations of the underlying muscle. The next effect, and possibly the first evident symptoms, if sweating and fasciculations do not occur or go unnoticed, are gastrointestinal-related: nausea and vomiting, with or without diarrhea. Mild dermal exposure may not produce effects for hours. Large amounts by either route produce a sudden loss of consciousness, seizures, flaccid paralysis, apnea, and death. The most likely cause of death will be from paralysis of the respiratory muscles and severe depression of the central nervous system (CNS).4
▪ PHYSIOLOGIC CONSIDERATIONS
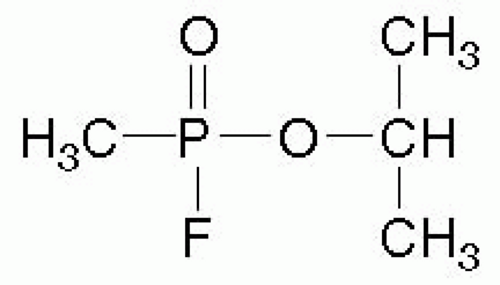
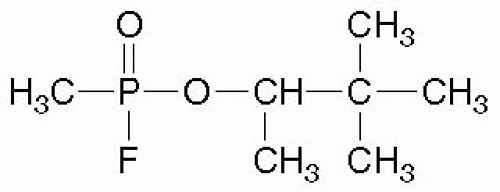
NA are potent organophosphate compounds that are similar to, but more toxic than, insecticides. NA include tabun (GA), sarin (GB), soman (GD), and V-agent (VX) (see Figs. 68.1 and 68.2). (The “G” designation was allegedly given because these agents were developed by Germany, and the letter “V” allegedly stands for venomous5). In 1994, when sarin was released by terrorists in Matsumoto, Japan and again in 1995 in Tokyo subways, 12 people were killed, and more than 4,000 sought treatment.6
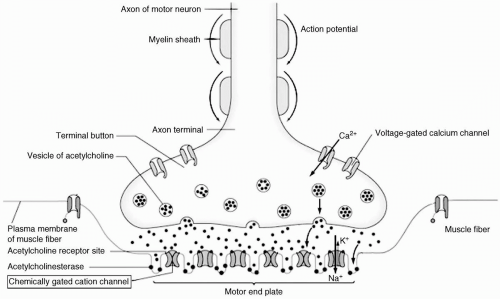
NA produce biological effects by inhibiting various types of acetylcholinesterase (AChE),4 which in turn prevents the hydrolysis of the neurotransmitter, acetylcholine, and subsequently leads to the overstimulation of organs with cholinergic receptors (see Fig. 68.3).7 Those of clinical importance include the exocrine glands, the smooth and striated muscles, and the nerves in the CNS and at the ganglia. There is no assay to directly measure the degree of exposure to NA. The degree of AChE impairment in tissue may be estimated by measuring the AChE activity in red blood cells.8
What Is the Clinical Management of Victims Afflicted by Nerve Gas?
Management consists of ventilatory support, administration of antidotes, and, for severe cases, an anticonvulsant.9 Ventilation may be difficult because of the intense bronchoconstriction. One antidote for mild exposure is atropine, 2 mg, given intramuscularly and repeated every 20 minutes until the patient is fully atropinized (characterized by the appearance of flushed and dry skin, increased heart rate, and reduced bronchoconstriction and bronchorrhea). The pediatric dose for mild exposures is intramuscular atropine, 0.02 mg per kg. In moderate to severe cases, the dose of atropine is increased to 2 mg, given intravenously every 5 to 10 minutes for adults and 2.0 mg or 0.02 to 0.1 mg per kg for children. Atropine works by inhibiting the effects of excessive acetylcholine at the synaptic site. Patients exposed to NA may require very large doses of atropine for treatment to be successful. Although this drug is crucial to the elimination of many of the symptoms seen in exposure to NA, it does nothing to stop the effects of the nerve agent on the AChE itself.4 For that, another agent is required, such as PAM, which reactivates the AChE by competing with the nerve agent for the active site on the AChE and allows the AChE to resume almost normal activity.10 The adult dosage of PAM for mild cases is 1 to 2 g, given intramuscularly as a single dose; the pediatric dose for mild cases, also given intramuscularly, is 15 to 25 mg per kg. For moderate to severe exposures, the dose for PAM is the same, but is usually given intravenously. It should be given over 30 minutes, because rapid administration may cause significant elevations in blood pressure, and doses should be repeated hourly in case of progressive worsening or persistent signs of toxicity.4
How Do Nerve Agents Affect the Neuromuscular Junction?
Time is of the essence when treating patients exposed to NA for reasons other than the obvious need for rapid medical attention. The bonds formed between the nerve agent and AChE will rapidly “age” and become resistant to deactivators right after exposure.11 Oximes are only therapeutically useful if the agent-enzyme complex has not aged.12 Complete aging may take minutes (soman) to hours (VX). Because, in most cases, the exact agent is unknown, PAM should be given for any exposure, regardless of the time transpired. Once AChE is irreversibly inactivated, it must be replaced by cellular production; this occurs at different rates in blood (RBC turnover is 1% per day) and tissue, in which it may take up to 6 weeks in patients who did not receive treatment.11,13 Owing to the inactivation of various AChE, drugs that depend on AChE for breakdown—such as succinylcholine, remifentanyl, esmolol and mivacurium—should not be used in patients exposed to NA.14
One of the other main areas affected by significant exposure to NA is the CNS; seizures are common and are an early indication of contact with the nerve agent. Intravenous benzodiazepines (e.g., diazepam, 10 mg) and scopolamine (0.25 mg every 4 to 6 hours for mild cases, and 0.25 mg, repeated in 30 minutes, followed every 4 to 6 hours for moderate and severe cases) can be used to suppress the CNS effects of NA. Scopolamine has a sedative effect in addition to a central anticholinergic effect due to its ability to penetrate the blood-brain barrier. Benzodiazepines can both stop and prevent seizures, while facilitating mechanical ventilation. Prophylaxis treatment with pyridostigmine given orally (30 mg t.i.d. for a population at risk) can be an effective, partially protective measure against nerve agent
intoxication.4,15,16 However, there are reports of possible long-term neuromyasthenic injury from pyridostigmine given on a recurring basis, including its potential role in Gulf War Syndrome.12,13,17
intoxication.4,15,16 However, there are reports of possible long-term neuromyasthenic injury from pyridostigmine given on a recurring basis, including its potential role in Gulf War Syndrome.12,13,17
How Do Toxic Gases Affect the Pulmonary System?
Pulmonary intoxicants are substances that damage the parenchyma of the lung. The damage caused by a gaseous substance usually manifests as pulmonary edema, although there may be some airway damage by several compounds in this category. In contrast, sulfur mustard damages the airway with little parenchymal damage. The best known and most studied of these compounds is phosgene (carbonyl chloride, designated as CG). Phosgene is widely used in industry, with hundreds of thousands of tons manufactured annually.
The first 30 minutes after exposure to low concentrations of phosgene may produce a mild cough, a sense of chest discomfort, and dyspnea. These compounds may also cause minor and transient irritation of the eyes and upper airways upon contact. In the lung, they damage the alveolar-capillary membrane and allow fluid to leak through. These effects are often delayed, and coughing (with production of clear, frothy fluid) may begin 2 to 72 hours after exposure. Generally, the shorter the onset time, the more severe the exposure and subsequent illness. The onset of pulmonary edema within 2 to 6 hours of contact is predictive of severe injury.5
There is no specific antidote for these compounds. Management consists of supportive care, primary pulmonary care, and assisted ventilation and oxygen until the lung parenchyma heals.18 Some cases develop rapidly and progress to acute respiratory lung disease (ARDS) or pulmonary edema, and require extensive lung-protective ventilation. Hypovolemia and hypotension may result from intravascular fluid loss into the lungs. Steroids have not been found to be useful in treating phosgene-induced lung damage.5
How Do Mustard Gas and Other Vesicants Work?
Vesicants are substances that cause vesicles, or blisters, and may be of animal, vegetable, or mineral origin. These agents include sulfur mustard, nitrogen mustard, lewisite (an arsenic agent), and phosgene oxime (not a true vesicant because it produces solid lesions). The one of most concern as a chemical weapon is sulfur mustard. This substance produced major casualties in World War I, when it caused more chemical casualties than all other chemical agents combined. It was also used extensively in the Iran-Iraq War in the 1980s.5
Mustard agents will cause significant damage in either vapor or liquid form.5 It damages and eventually kills cells, probably by disrupting DNA, although the exact mechanism is still unclear. It also damages organs and affects especially the eyes, skin, and lungs. Absorbed mustard damages bone marrow, lymphoid tissue, and gastrointestinal mucosa. The damage is similar to that produced by radiation. At first, contact with mustard agents causes no noticeable effects. After a brief period of 30 minutes of mild exposure, erythema may occur, accompanied by pruritus, burning, and tingling. After an onset time of 2 to 24 hours, the characteristic lesions appear on the skin. The eyes (the most sensitive organ to mustard exposure) become reddened, with possibly more severe damage to follow, and airway mucosa is damaged beginning in the upper airway, with a dosedependent descent. After large areas make contact with the offending agent, lesions may be characterized by a central zone of necrosis surrounded by blisters.19 Bone marrow damage from exposure to the mustard agent may lead to reduced resistance to infection. Mustard may be differentiated from other vesicant agents by the fact that other blistering vesicants, such as Lewisite, cause pain within minutes after contact, whereas mustard will not cause pain until the lesions appear. There is no antidote for exposure to mustard agents.20 The only effective means of preventing or decreasing damage after contact is rapid decontamination within 1 to 2 minutes. Management consists of relieving symptoms by the usual clinical measures, with emphasis on preventing infection. Health care workers must wear protective gear to avoid being contaminated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree