Glenn Ramsey
Blood component therapy is the mainstay of treatment for hemorrhagic shock, acute or chronic anemia, and acquired or congenital disorders in hemostasis. Anesthesiologists serve a unique role as perioperative physicians frequently charged with the management of patients suffering acute blood loss or coagulopathy. Therefore, it is important to understand the physiologic principles of oxygen delivery and hemostasis, the risks and safety precautions associated with blood-product transfusion, and the pharmacology of anticoagulants, antithrombotics, and procoagulant medications.
I. Blood-Product Transfusion
A. Component Therapy and Indications for Transfusion
Blood-product transfusion is conventionally performed with individual component therapy targeted to replace the specific deficiencies at hand. Whole fresh blood is occasionally used for critical bleeding at field hospitals for the military, but it is difficult to store and inefficient. Most patients require only a single blood component or a combination of selected components. For example, red blood cells (RBC) are needed to treat anemia with evidence of tissue hypoxia, whereas plasma is used to treat coagulopathy and factor deficiencies. Separating blood transfusion into component therapies allows for targeted efficient treatment while also minimizing the risks of transfusion reactions and transfusion transmitted infection.

There are few, if any, indications for transfusion of whole blood. Transfusion of separated blood components, such as RBCs or plasma, allows for targeted treatment and saves a precious resource.
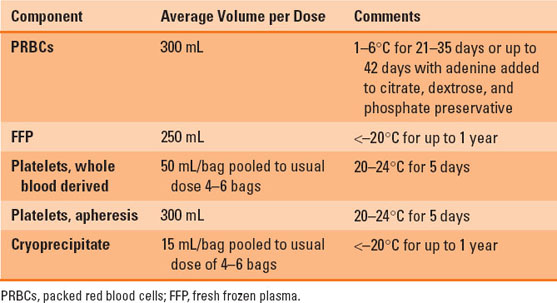
Packed red blood cells (PRBCs) are the most common blood product transfused worldwide, with over 13 million units administered annually in the United States (1). A unit of PRBCs is obtained from a single donor and consists of about 300 mL, with a hematocrit of approximately 70% and only about 20 to 30 mL of plasma. One unit of PRBCs generally increases the patient’s hemoglobin concentration by 1 g/dL. Table 24-1 presents the blood component storage and preparation details.
Table 24-1 Blood Component Storage and Preparation
The simplest indications for PRBC transfusion are acute blood loss or anemia with evidence of inadequate oxygen delivery to the tissues. Patients suffering hypovolemic shock secondary to critical bleeding clearly require resuscitation with PRBCs, but they may also require treatment of dilutional coagulopathy and thrombocytopenia. Typically, the hemoglobin transfusion threshold for active bleeding in hemodynamically unstable patients is higher (hemoglobin goals of >8.0 g/dL) to allow for reserve during active bleeding. Transfusion for major bleeding should also include replacement of coagulation factors and platelets with consideration given to administration of medications and strategies for blood conservation.

There is no general rule regarding the minimum hemoglobin at which patients should be transfused. The trigger for transfusion should be individualized for each specific patient.
The transfusion threshold for hemodynamically stable patients with anemia has been the topic of many review articles and original studies for over two decades. The controversy stems around the balance of the benefits of RBC treatment for anemia versus the risks of transfusion. Most international guidelines, including the AABB, formerly the American Association of Blood Banks, the American Society of Anesthesiologists, the British Committee for Standards in Haematology (BCSH), the Society for Thoracic Surgeons, and the Society of Cardiovascular Anesthesiologists, agree that restrictive transfusion practices are indicated for most hemodynamically stable trauma, perioperative, and critically ill patients (2). There are a few circumstances, such as severe sepsis, acute myocardial ischemia, and acute neurologic injury, where higher transfusion triggers are indicated to maximize oxygen delivery to end organs under stress. Otherwise, hemodynamically stable patients without evidence of tissue hypoxia (elevated lactate levels, low central venous oxygen saturation) generally tolerate hemoglobin levels as low as 7.0 g/dL with compensatory mechanisms to increase cardiac output and oxygen extraction at the tissue level. Figure 24-1 describes a suggested clinical algorithm adapted from the BCSH guidelines for management of anemia and RBC transfusion (2,3).
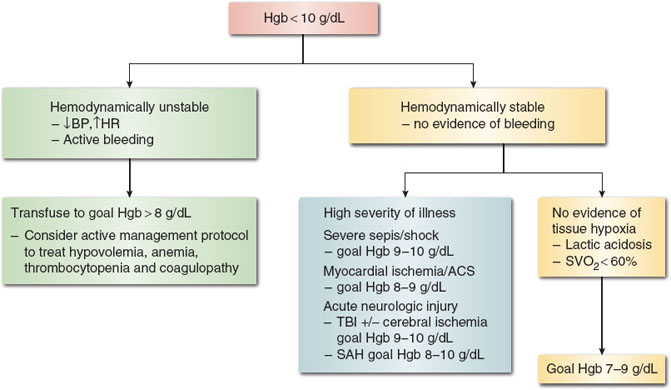
Figure 24-1 Suggested algorithm for red blood cell transfusion in hemodynamically stable and unstable patients. Stable angina and history of cardiovascular disease do not require transfusion thresholds greater than 7.0 g/dL. BP, blood pressure; HR, heart rate; ACS, acute coronary syndrome; TBI, traumatic brain injury; SAH, subarachnoid hemorrhage. (From Retter A, Wyncoll D, Pearse R, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. Br J Haematol. 2013;160(4):445–464, with permission.)
Fresh frozen plasma (FFP) contains all the clotting factors, fibrinogen, and plasma proteins from whole blood donation or apheresis. The volume of 1 unit of FFP is approximately 300 mL, with physiologic levels of stable clotting factors and approximately 70% of labile factors VIII and V. The most common indications for FFP are treatment of dilutional coagulopathy and factor deficiency. The recommended dose is 10 to 15 mL/kg. Other indications for FFP include replacement of antithrombin in cases of long-term heparin use or as a second-line agent for warfarin reversal.
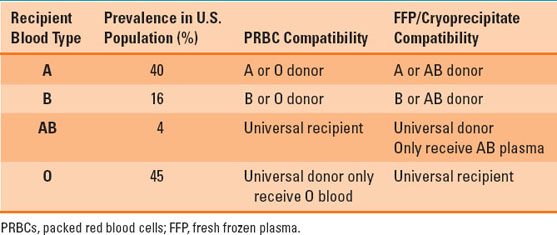
FFP contains the antibodies to blood type antigens and must therefore be compatible when transfused. Table 24-2 presents the FFP compatibility profiles as they compare with RBC component compatibility. The ABO blood system is the major carbohydrate-derived blood-borne antigen system that produces naturally occurring immunoglobulin-M (IgM) antibodies without the need for RBC exposure. Thus, ABO incompatibility for PRBCs or plasma-containing components carries significant risk of acute hemolytic transfusion reactions (discussed in depth below). Type AB plasma is the universal FFP donor as it does not contain any ABO blood cell antibodies, while type O patients are the universal recipient for plasma because there are no A or B antigens in type O blood.
 VIDEO 24-1
VIDEO 24-1
Blood Transfusion Compatibility
Table 24-2 ABO Blood Group Prevalence and Blood Component Compatibility
Cryoprecipitate (sometimes called simply cryo) is produced after a controlled thaw and centrifuge of frozen plasma. The yield from 1 unit of FFP is small. Therefore, a dose of cryoprecipitate is usually pooled from four to six separate donors. The resultant product is approximately 100 mL that contains a high concentration of fibrinogen relative to FFP, as well as clinically significant amounts of factor VIII, von Willebrand factor, factor XIII, and fibronectin. Von Willebrand disease, hemophilia, hypofibrinogenemia, and disseminated intravascular coagulopathy (DIC) are the most common indications for cryoprecipitate transfusion.
Platelets are produced either as a pooled unit from four to six whole blood donors or from a single apheresis donation. Unlike other blood components, they have a short shelf life of only 5 days, and they are stored at room temperature and therefore carry a higher risk of bacterial contamination. Typically a single dose of platelets (pool or apheresis) is expected to increase the platelet count initially by 25,000 to 30,000 per microliter. However, the response to platelet transfusion varies greatly depending on the indication, acuity, and systemic syndrome of the patient.
Platelet transfusion may be indicated when platelets are decreased as a result of dilution, bleeding, destruction, or sequestration. Transfusion thresholds for thrombocytopenia depend on whether the patient has clinical signs of bleeding or whether bleeding or the risk of bleeding involves the limited intraorbital, intracranial, or neuraxial spaces. In such instances, the transfusion threshold is <100,000 per microliter. Otherwise, for surgical patients where bleeding is anticipated and prophylaxis is desired, the threshold is generally <50,000 per microliter. Patients without clinical signs of bleeding are not at risk of spontaneous hemorrhage until the platelet count drops to <10,000 per microliter. Platelet transfusion may also be necessary for patients with qualitative deficiencies that are acquired or congenital. Commonly acquired platelet dysfunction occurs with extracorporeal circulation, such as cardiopulmonary bypass, or with medications or systemic illnesses, such as liver disease and uremia.
II. Blood Compatibility
RBC compatibility testing consists of typing for ABO and Rh(D), screening the plasma for non-ABO antibodies, and crossmatching prospective RBC units. Group O, A, and B persons have naturally occurring strong plasma anti-A and/or anti-B to the antigen(s) they lack, and RBC units must be ABO compatible to avoid hemolytic transfusion reactions. D-negative persons can easily make anti-D when exposed to D-positive RBCs and should normally receive D-negative RBCs. This is especially important for girls and young women to avoid risk of hemolytic disease of the newborn in future D-positive fetuses. One to 2% of all patients, and 5% to 20% of multitransfused patients have non-ABO hemolytic alloantibodies to Rh and other blood group antigens. These antibodies must be identified so that RBC units negative for the target antigens can be given to avoid hemolysis. After these “type and screen” tests, donor RBC units are crossmatched for the patient, either by computer confirmation or, if significant antibodies are present, by serological crossmatching of plasma versus donor RBCs. Compatibility testing routinely takes 45 to 60 minutes or longer if RBC alloantibodies, warm (IgG), or cold (IgM) autoantibodies are present. If RBCs must be given emergently before testing is completed, uncross-matched group O RBCs (D-negative for girls and young women) are the best choice, after weighing the risk of non-ABO hemolytic RBC antibodies. In an emergency, as a guide to help remember that group O is a “universal donor,” think of the “O” in donor.

In an emergency, when a bleeding patient’s blood type is not known and there is no time to perform a crossmatch, it is best to transfuse type O Rh-negative blood.
III. Blood Administration
Before transfusion, it is mandatory that the blood bank’s transfusion tag on the blood unit be carefully checked against the blood bag and the patient’s wristband identification to avoid a hemolytic reaction from administering the wrong blood or component. All blood components must be administered through a 150- to 260-μm blood filter to prevent clots from entering the patient’s bloodstream. Products should be infused within 4 hours of issuance from the blood bank. A blood warmer should be used in rapid large-volume transfusions to avoid hypothermia and may be recommended for transfusing RBCs to patients with cold autoantibodies.
IV. Transfusion Reactions
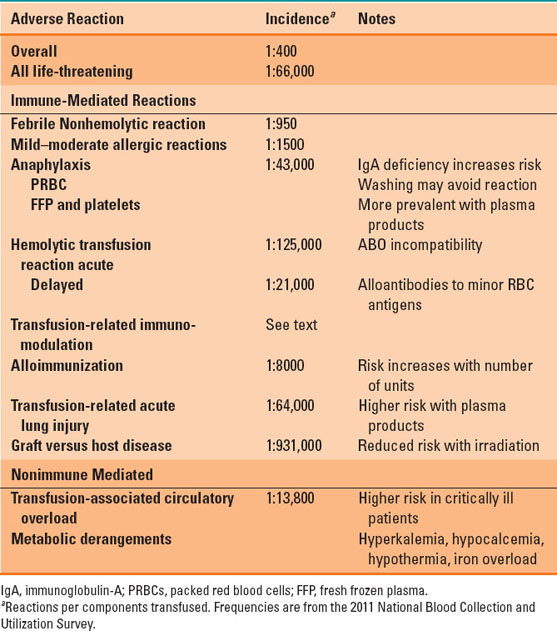
With over 20 million blood components transfused annually, the risks of transfusion are relatively rare, with an overall incidence of 0.24% or 2.4 reactions per 1,000 units transfused (1). More than half of these reactions are mild, febrile nonhemolytic reactions or mild to moderate allergic reactions. However, the overall incidence of transfusion reactions is likely underreported and significantly higher than published. Transfusion reactions are often organized by pathophysiology into immune-mediated or nonimmune-mediated reactions. The latter include transmission of infection (e.g., hepatitis C) or metabolic derangements associated with massive transfusion (e.g., hyperkalemia). Table 24-3 summarizes many of the reported noninfectious adverse effects of transfusion, but the following sections will focus on some of the most clinically significant reactions.

Acute hemolytic transfusion reactions most often result from errors made by medical personnel. It is critical that blood donors and recipients be properly identified and all labels on blood products be properly matched to those individuals.
Table 24-3 Noninfectious Transfusion Reactions
Hemolytic transfusion reactions result from intravascular or extravascular hemolysis of endogenous and transfused RBCs, typically when the recipient expresses antibodies to blood-borne antigens within the donor product. This reaction is acute and severe when transfusion involves the naturally occurring IgM anti-A and anti-B antibodies to the ABO blood cell antigens. Acute hemolytic transfusion reactions (AHTR) are rare and almost always result from clerical errors with blood sampling, typing, crossmatch, or erroneous administration of an inappropriate blood product to the wrong patient. Rarely, transfusion of incompatible plasma can also result in acute hemolysis (Table 24-2) (4).
Vigilance for the diagnosis of AHTR must remain high because many of the signs and symptoms can be masked during general anesthesia. Responsive patients may complain of itching, chest pain, or abdominal discomfort. Vital signs become unstable, with diffuse bradykinin and histamine release leading to fever, hypotension, tachycardia, and bronchospasm. AHTRs are best treated with supportive care after discontinuing all blood-product transfusions and initiating investigation into the etiology of the incompatibility. The mortality from AHTR remains high, accounting for up to 26% of the transfusion-related fatalities reported to the U.S. Food and Drug Administration from 2008 to 2012. Patients may progress to multiorgan system failure from systemic shock, DIC, acute renal failure, and obstructive hepatic dysfunction (5).
Delayed hemolytic transfusion reactions (DHTR) often occur days after blood-product administration and are typically less severe, presenting with progressive anemia, jaundice, and hemoglobinuria in the absence of hemodynamic instability. DHTR results from a humoral reaction to antigens in transfused blood products in recipients with a history of alloimmunization to antigens such as Rh, Kell, Kidd, Duffy, among others. Alloimmunization occurs with pregnancy or exposure to blood-product transfusion as the recipient develops antibodies to blood-borne antigens. This puts these patients at risk for future hemolytic transfusion reactions and emphasizes the importance of antibody screen and complete crossmatch prior to nonemergency transfusion.
Transfusion-related immunomodulation (TRIM) was originally described in the 1970s when it was observed that patients who received transfusion were less likely to reject solid organ transplants. The exact mechanism of immunomodulation is not clear. But it likely relates to the adverse pro-inflammatory effects of biologic response modifiers (lipid breakdown products, chemokines, leukotrienes) included in blood-product components as well as the anti-inflammatory cell-mediated response of white blood cells included in blood component transfusions containing plasma. TRIM presumably occurs with every transfusion but may be limited with leukoreduction or irradiation. Recent reviews in transfusion medicine point to the adverse immune response as the etiology for morbidity and mortality with liberal transfusion strategies (4).
Transfusion-related acute lung injury (TRALI)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








