Arrhythmia
Judy Cheng PharmD, BCPS
An arrhythmia is defined as a disturbance of cardiac electrical conduction. The synchronous interaction between the electrical and the mechanical properties of the heart is important for the heart to function properly and to maintain adequate blood supply and perfusion to other vital body organs. The management of arrhythmias continues to evolve as results of many landmark studies are published.
With the original discovery of surface electrocardiogram (ECG) and the more recent availability of intracardiac recordings and programmed cardiac stimulation, there is considerable insight into cardiac electrophysiology. This technology has allowed for sophisticated classification of many arrhythmias.
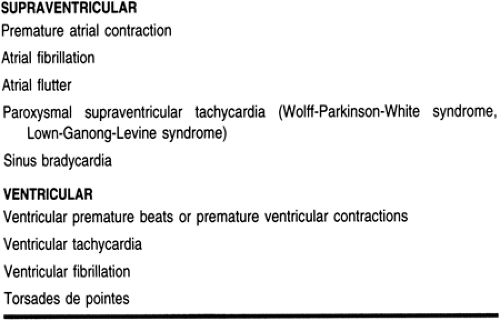
Cardiac arrhythmias can be broadly classified into two groups: supraventricular (originate above the ventricle) and ventricular (originate from the ventricle) arrhythmias. Common supraventricular arrhythmias include atrial fibrillation, atrial flutter (Aflutter), paroxysmal supraventricular tachycardia (PSVT), sinus tachycardia, and bradycardia. Atrial fibrillation is probably the most common sustained cardiac arrhythmia encountered in primary care practice. The prevalence of atrial fibrillation increases with increasing age and the presence of structural heart disease. Atrial fibrillation is the major cause of stroke, especially in the elderly. Ventricular arrhythmias include ventricular premature beats (VPBs or PVC), monomorphic and polymorphic (such as torsades de pointes) ventricular tachycardia (VT), and ventricular fibrillation (VF).
Multiple antiarrhythmic agents are available for management of these disorders. Unfortunately, many problems are associated with these agents, proarrhythmia being the most significant since it has a potential effect on patient mortality. Fortunately, technical advances have been made in the development of nondrug therapies. Some examples include radiofrequency ablation, the internal cardioverter/defibrillator, and pacemakers.
This chapter will discuss the pathophysiology, management and social impact of arrhythmias in a primary care setting, with an emphasis on long-term management to prevent complications such as life-threatening ventricular arrhythmias, heart failure, or thromboembolic events.
ANATOMY, PHYSIOLOGY, AND PATHOLOGY
Anatomy and Physiology
CARDIAC ELECTRICAL CONDUCTION PATHWAY
The heart is an organ that is capable of producing an intrinsic electrical rhythm and has automaticity. This rhythm may be modified by the autonomic nervous system (sympathetic and parasympathetic nervous systems). In normal cardiac conduction, the electrical activity begins in the sinoatrial (SA) node, or the pacemaker of the heart. The SA node is located in the upper portion of the right atrium (Fig. 7-1).
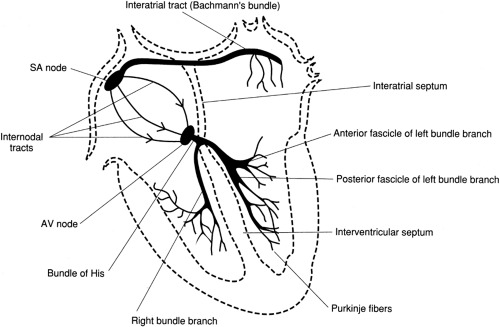 FIGURE 7-1 The electrical conduction pathway of the heart. ( Huff, J. [1997]. ECG Workout: Exercises in Arrhythmia Interpretation, 3/e. Philadelphia: Lippincott-Raven Publishers. ) |
In adults, the SA node fires at a regular rate of 60 to 100 beats per minute (bpm), which is defined as the normal sinus rhythm (NSR). Following discharge of the SA node, the electrical impulse is conducted through the right atrium, to the left atrium, then to the atrioventricular (AV) node. The impulse continues to conduct through the AV node, with a momentary delay, then to the bundle branches. From the bundle branches, the electrical activity is carried to the ventricular muscle by networks of Purkinje fibers. In abnormal cardiac conduction, enhanced automaticity and formation of re-entry circuits at certain foci of the heart (either SA node or other latent pacemakers) may lead to tachycardia.
Electrical impulses are capable of conduction through ion channels. The major ion channels include sodium and potassium channels (fast conduction channels), as well as calcium channels (slow conduction channels). They are labeled as fast and slow conduction channels based on the speed of ion influx and efflux. These conduction channels are distributed throughout the myocardium. However, most sodium and potassium channels are located in the atria and ventricular muscle, and most calcium channels are located at the SA and AV nodes respectively. The activity of antiarrhythmic agents is based on these sites of action. For instance, calcium channel blockers such as verapamil will exert their main action at the SA and the AV nodes. Class I antiarrhythmic agents, such as procainamide are sodium channel blockers, exert their action mainly on the atrial and the ventricular tissue.
ELECTROCARDIOGRAM
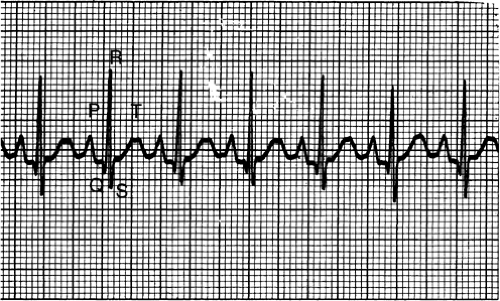
The electrocardiogram (ECG) complexes reflect the cardiac electrical activity. Electrical conduction of the heart is represented by waveforms on the ECG. The P wave represents atrial depolarization, the QRS complex represents ventricular depolarization and the T wave represents ventricular repolarization (Fig. 7-2). The atrial contraction follows the P wave and the ventricular contraction follows the QRS complex. There are three key intervals that play an important part in ECG interpretation. The PR interval, normally 0.12 to 0.20 seconds in duration, represents the travel of electrical impulse from the SA node through the AV node. A prolongation of the PR interval indicates a delay in AV nodal conduction, known as first-degree
heart block. The QRS interval, normally less than 0.12 seconds in duration, represents the time required for the ventricle to depolarize. Prolongation of the QRS interval may indicate ventricular hypertrophy (it takes longer for a thicker piece of muscle to depolarize) or a defect in the conduction of the Purkinje fibers. The QT interval, normally 0.4 seconds or less, represents the time necessary for ventricular depolarization and repolarization. Prolongation of the QT interval (> 0.5 seconds) indicates prolongation of the relative refractory period. During this phenomenon the ventricles are especially vulnerable to extra stimuli. This increases the risk of developing life-threatening arrhythmias such as torsades de pointes.
heart block. The QRS interval, normally less than 0.12 seconds in duration, represents the time required for the ventricle to depolarize. Prolongation of the QRS interval may indicate ventricular hypertrophy (it takes longer for a thicker piece of muscle to depolarize) or a defect in the conduction of the Purkinje fibers. The QT interval, normally 0.4 seconds or less, represents the time necessary for ventricular depolarization and repolarization. Prolongation of the QT interval (> 0.5 seconds) indicates prolongation of the relative refractory period. During this phenomenon the ventricles are especially vulnerable to extra stimuli. This increases the risk of developing life-threatening arrhythmias such as torsades de pointes.
Pathophysiology
The mechanisms of tachyarrhythmias (both supraventricular and ventricular) have been classified into two general categories; those resulting from an abnormal impulse generation and those resulting from an abnormal impulse conduction or reentrant tachycardia.
Automatic tachycardias depend on spontaneous impulse generation in latent pacemakers, which compete with the SA node for dominance of cardiac rhythm. If the rate of spontaneous impulse generation of the abnormally automatic tissue exceeds that of the SA node, then an automatic tachycardia may result. Trigger automaticity is also a possible mechanism for abnormal impulse generation. Trigger automaticity is the transient membrane depolarization that occurs during repolarization (early after-depolarization) or after depolarization (delayed after-depolarization) but before repolarization.
Reentry tachycardia involves indefinite propagation of the impulse and continued activation of previously refractory tissue. Three conduction requirements must be fulfilled for the formation of a viable reentrant focus. There must exist two pathways for impulse conduction with an area of unidirectional block (prolonged refractoriness) and slow conductions in the other pathway (Fig. 7-3).
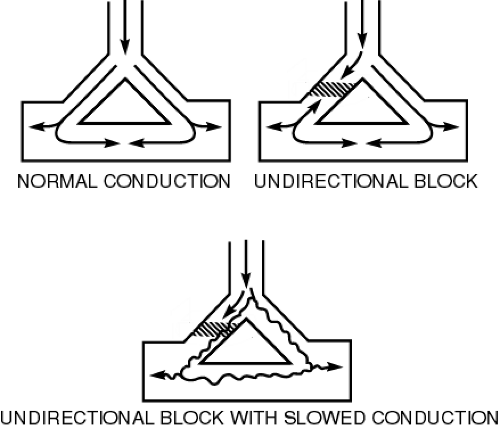 FIGURE 7-3 Mechanism of re-entry. ( Bauman, J. (1983). Understanding and treating supraventricular arrhythmias. Clinical Pharmacology Therapeutics. 2:312–320. ) |
A critically timed premature beat initiates reentry. The premature impulse enters both conduction pathways but encounters refractory tissue in one of the pathways at the area of the unidirectional block. The impulse subsides because it is still refractory from the sinus impulse. Although it fails to propagate in one pathway, the impulse will still proceed in a forward (antegrade) direction through the other pathway because of this pathway’s relatively shorter refractory period. The impulse may then proceed through the loop of tissue and reenter the area of the unidirectional block in a backward (retrograde) direction. Because the antegrade pathway has slow conduction properties, the area of unidirectional block has time to recover its excitability. The impulse can proceed retrogradely through this (previously refractory) tissue and continue around the loop of tissue. The reentrant focus may excite surrounding tissue at a rate
greater than that of the SA node, and a clinical tachycardia results. Bradycardia is usually due to sick sinus or AV nodal conduction delay or blockade.
greater than that of the SA node, and a clinical tachycardia results. Bradycardia is usually due to sick sinus or AV nodal conduction delay or blockade.
Different precipitating factors of automaticity and reentry may lead to different types of bradyarrhythmias or tachyarrhythmias. Classifications of cardiac arrhythmias are summarized in Table 7-1. Pathophysiology of some of the more clinically significant arrhythmias are discussed in greater detail below.
SUPRAVENTRICULAR ARRHYTHMIA
Atrial Fibrillation and Atrial Flutter
In atrial fibrillation, reentry circuits are formed when the atrium is being stretched or disturbed. They take over the SA node and become multiple independent pacemakers of the heart. All of these pacemakers beating independently results in an overall atrial rate of 400 to 600 bpm. In atrial flutter, the mechanism is similar except that there is one instead of multiple reentry circuits.
Paroxysmal Supraventricular Tachycardia
Paroxysmal supraventricular tachycardias (PSVTs) include those arrhythmias caused by AV nodal reentry, SA nodal reentry, and intra-atrial reentry. AV nodal reentry is by far the most common form of PSVT. Lown-Ganong-Levine (LGL) syndrome is an example of AV nodal reentry PSVT. Wolff-Parkinson-White (WPW) syndrome is another type of PSVT that involves both the AV node (slow conduction pathway) and an extranodal AV connection (accessory fast conduction pathway). PSVT that involve accessory pathway may be the more common orthodromic (down AV node, up accessory pathway) or the less common antidromic (down accessory pathway, up AV node) type.
VENTRICULAR ARRHYTHMIAS
Ventricular Tachycardia
Ventricular tachycardia is defined as three or more repetitive VPBs, occurring at a rate greater than 100 bpm. VT can be subdivided into two major types, monomorphic VT which has a consistent QRS morphology, and polymorphic VT, which has varying QRS morphology. VT may occur acutely as a result of metabolic abnormalities, electrolyte abnormalities such as hypokalemia, ischemia, organic heart disease such as congestive heart failure (CHF), left ventricular aneurysm, or drug toxicities such as digoxin overdose. Ventricular tachycardia, if sustained for a prolonged period of time, can lead to hemodynamic collapse. Immediate treatment is required.
Ventricular Fibrillation
Ventricular fibrillation is an electrical anarchy of the ventricle, resulting in no blood pressure or cardiac output. VF is a medical emergency that requires advanced cardiac life support measures. VF is often preceded by VT. Ventricular fibrillation occurs most commonly in patients with ischemic heart disease, WPW, and less commonly, mitral valve prolapse.
Torsades de Pointes
Torsades de pointes is a rapid form of polymorphic VT associated with delayed ventricular repolarization (prolonged QT intervals). Table 7-2 lists the common causes of Torsades.
EPIDEMIOLOGY
The most common type of arrhythmia, other then atrial and ventricular premature contraction (which most people will experience during their lifetime), is atrial fibrillation. The best source of epidemiology data on atrial fibrillation is the Framingham study (Kannel et al, 1982; Wolf et al, 1987). It is important to realize that the Framingham population may not represent the ethnic and racial diversity in other parts of the country.
Among patients in the Framingham Heart Study, the cumulative 22-year incidence of new atrial fibrillation was 21.5 per 1000 men and 17.1 per 1000 women respectively. However, this incidence rose sharply with increases in age, from 0.5% for age 50 to 59 years to 8.8% for age 80 to 89 years. Excluding people with rheumatic heart disease, the 2-year incidence of developing atrial fibrillation in the Framingham study was 0.04% for men and 0% for women age 30 to 39 years but rose to 4.6% for men and 3.6% for women age 80 to 89 years. Heart failure and rheumatic heart disease were the most powerful predictive precursors for atrial fibrillation, with relative risk in excess of sixfold. Hypertension, a history of cerebrovascular disease, diabetes, and left ventricular hypertrophy were also found to be significantly associated with atrial fibrillation.
The clinical importance of atrial fibrillation, other than its difficult-to-tolerate signs and symptoms, such as palpitation,
dizziness, and fatigue, is its relation to a mortality rate doubled that of control subjects. Much of its morbidity and mortality related to atrial fibrillation is caused by stroke (Wolf et al, 1987). This is because atrial fibrillation creates turbulent flow in the cardiac chambers, promoting the formation of thrombi. Such thrombi, when dislodged from the cardiac chambers, may eventually lodge in another organ and lead to a thromboembolic event. Other morbidity and mortality related to atrial fibrillation include heart failure. The risk of stroke from atrial fibrillation is estimated to be 1.5% for those age 50 to 59 years and approaches 30% for those age 80 to 89.
dizziness, and fatigue, is its relation to a mortality rate doubled that of control subjects. Much of its morbidity and mortality related to atrial fibrillation is caused by stroke (Wolf et al, 1987). This is because atrial fibrillation creates turbulent flow in the cardiac chambers, promoting the formation of thrombi. Such thrombi, when dislodged from the cardiac chambers, may eventually lodge in another organ and lead to a thromboembolic event. Other morbidity and mortality related to atrial fibrillation include heart failure. The risk of stroke from atrial fibrillation is estimated to be 1.5% for those age 50 to 59 years and approaches 30% for those age 80 to 89.
The phase “lone atrial fibrillation” refers to atrial fibrillation in the subgroup of patients who do not have other underlying cardiac diseases. This type of atrial fibrillation accounts for 11.4 percent of all cases of atrial fibrillation. Studies have indicated that prognosis in this group of patients is good (Kopecky, 1987).
For ventricular arrhythmias, the incidence of VT is not as well defined. Drug-induced torsades occurs in approximately 5% to 20% of patients (McCollam & Parker, 1991). Approximately 2% to 11% of patients will experience VF within 24 hours after myocardial infarction (MI) (Bauman & Dekker Schoen, 1997).
DIAGNOSTIC CRITERIA
Supraventricular Arrhythmias
PREMATURE ATRIAL COMPLEXES
A premature atrial complex (PAC) is an electrical impulse originating in the atria other than the sinus node. Premature atrial complexes often occur without apparent cause and symptoms. Commonly recognized clinical causes include the use of stimulants such as caffeine, tobacco, or alcohol.
The ECG criteria are:
Rhythm: Irregular
P waves: Different in morphology from those of sinus mode origin.
PAROXYSMAL SUPRAVENTRICULAR TACHYCARDIA
Paroxysmal supraventricular tachycardia is a clinical syndrome characterized by repeated episodes of atrial tachycardia with an abrupt onset (preceded frequently by a premature atrial contraction), lasting from a few seconds to many hours. They usually end abruptly and often can be terminated by vagal maneuvers. Possible causes of PSVT include physical and psychological stress, hypoxia, hypokalemia, and excessive use of caffeine or other stimulants. Primary cardiac causes include MI, congenital heart disease, and cardiomyopathy. Other causes include digitalis toxicity and chronic pulmonary disease. Patients may feel a sudden rapid palpitation and severe anxiety. Congestive heart failure, angina, and shock may occur as a result of a decreased cardiac output and an increased need for myocardial oxygen, if the tachyarrhythmia is persistent.
The ECG criteria are:
Rate: The atrial rate is usually 160–240 beats/minute.
Rhythm: Regular. The ventricular rate is most often regular with 1:1 AV conduction when the atrial rate is less than 200. When atrial rates above 200, 2:1 AV block is common. Higher-grade block may also occur.
P wave: P waves may be difficult to identify because they can be buried in the preceding T wave. However, when visualized, their morphology is different from the sinus P waves.
Lown-Ganong-Levine (LGL) Syndrome
Lown-Ganong-Levine, one type of PSVT, is usually benign unless debilitating tachyarrhythmias occur. ECG criteria are normal except PR intervals are abnormally short but constant.
Wolff-Parkinson-White (WPW) Syndrome
WPW occurs mostly in younger patients (20–35 years old). It usually requires no intervention unless debilitating tachyarrhythmias occur.
ECG criteria are:
Rate and rhythm: Within normal limits, however, WPW can predispose abrupt episodes of PSVT, atrial fibrillation and atrial flutter with rates up to 300 bpm.
PR interval: Short
QRS interval: Duration is prolonged. Beginning of the waveform is slurry (delta wave).
ATRIAL FIBRILLATION/ATRIAL FLUTTER
Atrial fibrillation is a type of supraventricular arrhythmia defined as an extremely rapid and disorganized atrial activation (400–600 atrial bpm). This occurs when other ectopic foci in the atrium take over the role of the sinus node as the pacemaker. Such disorganized atrial contractions lead to a supraventricular impulse penetrating the AV conduction system in variable degrees, resulting in a totally irregular, often rapid ventricular rate. Because the AV node is composed of mostly slow conduction channels compared to the atrial tissue, which is composed of
mostly fast conduction channels, most of the supraventricular impulses are not conducted through the AV node. Therefore, although the atrial rates usually vary between 400 to 600 bpm, the ventricle responds at a considerably slower rate (120–180 bpm).
mostly fast conduction channels, most of the supraventricular impulses are not conducted through the AV node. Therefore, although the atrial rates usually vary between 400 to 600 bpm, the ventricle responds at a considerably slower rate (120–180 bpm).
Atrial fibrillation is now believed to occur under certain distinct clinical circumstances:
As a secondary arrhythmia in the absence of structural heart disease but in the presence of systemic abnormalities that predispose the patient to atrial fibrillation
As a secondary arrhythmia associated with cardiac diseases that affects the atria
As an idiopathic primary arrhythmia (lone atrial fibrillation)
The etiology of atrial fibrillation is categorized in Table 7-3 (Geraets & Kienzle, 1993).
Any situation that leads to an increase in atrial mass, atrial dilatation (in fluid overload states), myocardial injury (in coronary artery disease), increased vagal tone, or any other disturbance to the atria (open heart surgery) increases the risk of developing atrial fibrillation.
Atrial flutter is another form of supraventricular arrhythmia. Atrial flutter occurs less frequently than atrial fibrillation but has similar precipitating factors, consequences, and approach to treatment. This arrhythmia is characterized by rapid but regular atrial activation (250–350 bpm). This slower and regular atrial activity leads to a regular ventricular response and a pulse rate in dividends of 300 bpm (100 bpm for a 3:1 AV conduction, 150 bpm for a 2:1 AV conduction, and 300 bpm for a 1:1 conduction). Atrial flutter usually occurs in conjunction with atrial fibrillation; in this situation, the ventricular rate will be irregular.
Symptoms of atrial fibrillation and atrial flutter are similar. Patients often present complaining of palpitation, dizziness, fatigue, and dyspnea, especially if they have a rapid ventricular rate. Patients with a relatively slower ventricular rate may be asymptomatic. A prolonged uncontrolled ventricular rate may lead to cardiac decompensation or CHF. Atrial fibrillation is usually categorized as paroxysmal or chronic and the management approaches are different. Atrial flutter, on the other hand, is an unstable rhythm and rarely remains chronic.
ECG criteria for atrial fibrillation are:
Rate: Atrial rate usually greater then 400 bpm. Ventricular rates usually range from 100 to 150 bpm but can be less than 100 bpm.
Rhythm: Irregularly irregular
P wave: No P wave
ECG criteria for atrial flutter are:
Rate: Atrial rate is rapid and around 250 to 350 bpm. Ventricular rate depends on degree of AV block (usually between half to one quarter of the atrial rate.
Rhythm: Regular
P wave: Saw-toothed, referred to as “flutter wave”
BRADYARRHYTHMIAS
Bradyarrhythmia usually refers to sinus bradycardia. Sinus bradycardia is an arrhythmia where the sinus rate is below 60 bpm and all impulses come from the SA node. Sinus bradycardia is usually observed in athletes, most likely due to there increase in vagal tone. It is also noted during sleep, elevation of intracranial pressure, and situations with increased vagal tone such as straining of stool, vomiting, intubation, sick sinus syndrome, and hypothyroidism. Certain medications such as beta-blockers, calcium channel blockers and adenosine may also induce sinus bradycardia.
Ventricular Arrhythmias
VENTRICULAR PREMATURE BEATS (VPBs)
Ventricular premature beats are ectopic ventricular beats that occur earlier than the normally expected beat and is often followed by a compensatory pause. VPBs may be uniform, arising from the same ectopic focus (unifocal), or multiform (multifocal), arising from two or more different ventricular sites. Patients experiencing VPBs may be asymptomatic or may complain of “skipping beats.” Depending on a patient’s ventricular function and the duration and frequency of the VPBs, a decrease in cardiac output may result. VPBs are more dangerous if they occur in the presence of heart disease. Possible cause of VPBs include digitalis toxicity, hypokalemia, hypocalcemia, and the use of caffeine and other stimulants.
The ECG criterion is a QRS interval that is wide and bizarre in morphology. While most VPBs are benign, certain forms of VPBs may be predictive of potentially serious arrhythmic events (eg, sustained VT). Potentially dangerous VPBs include:
Couplets, triplets, or more VPBs in a row
Bigeminy (every other beat is a VPB) and trigeminy (every third beat is a VPB)
Multifocal VPBs (VPBs with different morphology)
More than six VPBs in a minute
R-on-T phenomenon: The VPB falls on the preceding beat’s T wave. This type of VPB can lead to VT and VF.
VENTRICULAR TACHYCARDIA
Ventricular tachycardia is defined as premature ventricular complexes occurring in succession at a rate of more than 100 bpm. If sustained, cardiac output may drop drastically and the rhythm may deteriorate to VF. Common cardiac causes of VT include acute MI, coronary artery disease, rheumatic heart disease, and cardiomyopathy or heart failure. Other noncardiac causes include pulmonary embolism, electrolyte imbalance (eg, hypokalemia) and drug toxicity (eg, digitalis, procainamide, quinidine, and vasopressors such as epinephrine). If conscious, the patient may complain of palpitations, dizziness, and shortness of breath. Patients usually lose consciousness quickly if VT is rapid and sustained.
ECG criteria are:
Rate: Atrial rate cannot be determined. Ventricular rate usually betwen 100 to 250 bpm.
Rhythm: Usually regular; slight irregularity may occur.
QRS complex: wide and bizarre.
VENTRICULAR FIBRILLATION
Ventricular fibrillation is an arrhythmia marked by rapid, disorganized depolarizations of the ventricles and characterized by a lack of organized electrical impulse, conduction, and ventricular contractions. Patients at risk of developing VF include those with acute MI, untreated VT, electrolyte imbalance (hypokalemia and alkalosis, hyperkalemia and hypercalcemia), electric shock, and hypothermia. Patients are usually unconscious in this situation and there is an absence of pulse, heart sounds and blood pressure. ECG criteria are an indeterminate rate and a rapid and chaotic rhythm, with no pattern or regularity.
TORSADES DE POINTES
Torsades de pointes is a syndrome characterized by atypical polymorphic VT (the QRS polarity spiral around the isoelectric line) and a prolonged QT interval. Any condition that lead to prolonged QT intervals can precipitate torsades (see Table 7-2). Similar to VT, patients with torsades usually lose consciousness rapidly. If conscious, the patient may complain of palpitations, dizziness, chest pain, or shortness of breath.
ECG criteria are:
Rate: Atrial rate cannot be determined, ventricular rate varies between 150 to 250 bpm.
QRS interval: Usually wide with a phasic variation in its electrical polarity, shown by complexes that point downward for several beats, then upward and so on.
QT intervals of the sinus beat preceding torsades are usually prolonged.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree