ACUTE GASTROINTESTINAL HEMORRHAGE
GENERAL CONSIDERATIONS, DEFINITIONS, AND EPIDEMIOLOGY
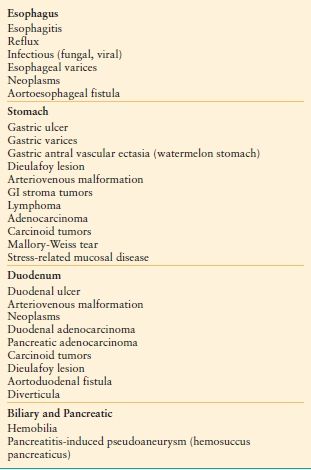
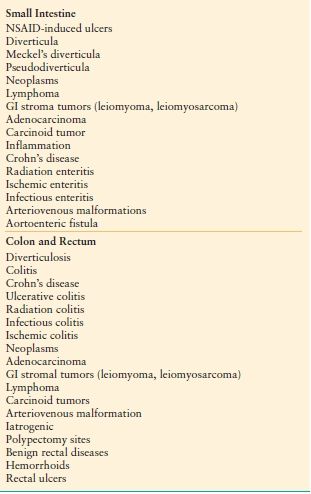
Gastrointestinal (GI) hemorrhage is common and remains an important cause of morbidity and mortality. The differential diagnosis of acute GI hemorrhage includes a large number of conditions and lesions, which are listed in Tables 34.1 and 34.2. The implications for the surgeon vary and depend upon the cause, location, and severity of the hemorrhage. Bleeding may be the initial manifestation of an underlying condition that requires surgery in its own right (typically GI tract malignancy). In other cases, surgery may only be required to control hemorrhage and in increasingly rare situations, may be required to control active bleeding and also treat the underlying disease (recalcitrant peptic ulcer disease). This chapter will focus on bleeding of recent onset and duration that may result in hemodynamic instability and the need for blood transfusion.
TABLE 34.2
CAUSES OF ACUTE MID AND LGIH
Many lesions cease bleeding spontaneously and others are so effectively treated with endoscopic methods that they rarely require surgical therapy. That surgical therapy is not often needed for the majority of GI bleeding has likely led to a general lack of experience on the part of surgeons in their management. Given that when surgery is needed, it is often emergent and patients are often critically ill, there is little room for error. With this in mind, this chapter aims to address GI hemorrhage as it is encountered by surgeons and discusses important components of nonoperative and operative management in general and where important, for specific sources of bleeding.
Bleeding has historically been categorized as “upper” or “lower” depending upon its origin relative to the ligament of Treitz. However, recent diagnostic advances (double-balloon enteroscopy and capsule endoscopy) have lead to a reevaluation of this classification scheme, in part, because small intestinal sources can now be directly visualized, which allows them to be classified separately from colonic sources.1,2 Upper GI hemorrhage (bleeding from the esophagus, stomach, or duodenum) accounts for 80% of cases of acute GI blood loss. Lower intestinal bleeding (colon and rectum) is responsible for 15%–20% of cases. Small intestinal lesions are responsible for <1% of cases in adults. Although difficult to estimate, approximately 100 hospital admissions per 100,000 persons in the United Kingdom were related to GI hemorrhage in 1989. The incidence of both upper and lower GI hemorrhage increases markedly with age.3
Patients with overt GI bleeding typically present to the hospital after an episode of hematemesis (the vomiting of blood), melena, or hematochezia. Melena is a black, tarry stool due to bacterial degradation of blood and can be evident after a 100 mL hemorrhage. Bleeding from the small intestine or right colon may also appear black. Hematochezia is the passage of bright red blood from the rectum and is usually indicative of a lower GI source. However, it may also be present in cases of massive upper GI hemorrhage. Patients with acute GI bleeding may present with the hemodynamic consequences of hemorrhage, including orthostatic syncope or near-syncope, complaints of dizziness, lightheadedness, and shortness of breath or palpitations.
History and physical examination can provide important clues to the cause and severity of bleeding. For example, melena after several days of worsening epigastric pain suggests peptic ulcer disease; whereas hematemesis or melena following vomiting or retching strongly suggests a Mallory-Weiss tear. Massive, painless upper GI hemorrhage in a patient with cirrhosis suggests bleeding from gastroesophageal varices, although other causes are responsible for bleeding in a significant number of patients with chronic liver disease.
A systematic physical examination is aimed at estimation of the magnitude of bleeding and the patient’s ability to compensate. Signs and symptoms of hypovolemia include cool, clammy, mottled skin, tachycardia, tachypnea, collapsed jugular veins, oliguria, and perhaps hypotension. Advanced age, concomitant medical conditions, and their treatment (β-adrenergic blockade) can obscure these physical findings. Physical examination should also document evidence of cirrhosis and portal hypertension, and rectal examination is mandatory and may demonstrate bright red blood or melena.
INITIAL RESUSCITATION AND OVERALL DIAGNOSTIC APPROACH
Many patients will require intravenous fluid resuscitation. The need for two large-bore peripheral intravenous lines is determined by the estimated blood loss based upon history and physical examination. Most bleeding stops spontaneously and intravenous crystalloid resuscitation may be all that is required. However, in all cases, blood is drawn for type and crossmatch, complete blood count with platelet count, serum electrolyte, glucose, BUN and creatinine concentrations, liver function tests, and coagulation profile.
Patients presenting with hypotension or evidence of impaired end organ perfusion (oliguria, confusion, cardiac ischemia) should receive blood and blood products early. There are no data to support a target hemoglobin concentration, and the goal is to achieve hemodynamic stability and restore tissue perfusion and oxygen delivery rapidly. The observation that transfusion of stable critically ill patients only when hemoglobin concentration drops below 7 mg/dL is safe cannot be applied to patients with active GI hemorrhage who were excluded from the study.4 In patients with active GI hemorrhage, a lower hemoglobin concentration was found to correlate with elevated troponin concentrations with several patients having chest pain to indicate ischemia.5 In summary, blood transfusion should be used when there is evidence of hemodynamic compromise and not based upon predefined transfusion thresholds.
Recently, resuscitation strategies have focused on an earlier and more balanced use of red blood cells (RBCs), plasma and platelet transfusions, and a relatively more restricted use of crystalloids. This approach has been considered beneficial primarily in the context of traumatic hemorrhage requiring massive resuscitation, but seems applicable to patients with GI bleeding who also require multiple units of packed RBCs. It is particularly important to correct coagulopathy and thrombocytopenia with plasma and platelets. Platelet transfusion can also be considered in patients taking aspirin or other nonsteroidal anti-inflammatory agents who may have impaired platelet function. However, data to support this practice is limited and platelet transfusion is not routinely recommended.6,7
Careful hemodynamic monitoring is vital to successful management. Patients who are actively bleeding and those who have sustained substantial hemorrhage should be admitted to an intensive care unit for close hemodynamic monitoring. The presence of chronic comorbidities, such as cardiac, renal, hepatic, or pulmonary disease, increases the risk of death and requires close observation, ideally in an intensive care unit. Bladder catheterization to monitor urine output, continuous transcutaneous oxygen saturation monitoring, frequent measurement of heart rate and blood pressure and the assessment of mental status are required. Invasive monitoring (central venous pressure and oxygen saturation monitoring, pulmonary artery catheter, arterial catheter for continuous pressure and blood gas monitoring) may be helpful in certain situations. However, none have been clearly demonstrated to improve outcomes in patients with GI hemorrhage. An organized system for caring for patients with GI hemorrhage that includes observation in a dedicated intensive care unit and management by a multidisciplinary team has been shown to improve processes of care and, in some studies, to improve outcomes.8,9
Flexible endoscopy will identify the source of bleeding in the majority of cases and is the mainstay for the evaluation of acute GI hemorrhage. Upper endoscopy (esophagogastroduodenoscopy [EGD]) should also be considered as the initial test in patients presenting with hemodynamic instability and hematochezia, as 13% of patients presenting with maroon stools or bright red blood have an upper gastrointestinal (UGI) source.10 Nasogastric saline lavage and aspiration with bloody return confirms an UGI source. However, a non-blood effluent does not reliably eliminate an UGI source nor does it confirm cessation of bleeding. Timely colonoscopy can identify the source of lower gastrointestinal hemorrhage (LGIH) in the majority of cases. Thorough cleansing of the colon facilitates visualization of mucosal lesions, and improves diagnostic accuracy. For this reason, colonoscopy is generally delayed to allow mechanical cleansing with a large volume polyethylene glycol-based solution.
Prognostic Factors and Scores
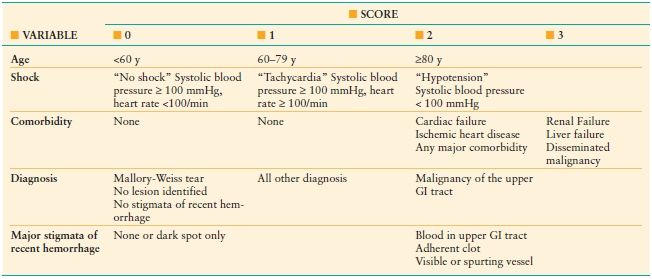
Several classification systems have been developed and tested for their ability to characterize a patient’s risk for rebleeding and for death after GI hemorrhage. Prognostic systems for upper GI hemorrhage have been more widely adopted than those for bleeding from the lower GI tract. The Rockall score was developed to estimate the risk of death in patients with upper GI hemorrhage and is summarized in Table 34.3. A clinical or preendoscopic Rockall score can be assigned and is useful to identify patients at low risk who can be managed as outpatients, without early endoscopy.11 The addition of endoscopic findings increases the accuracy of mortality predictions. The score can also be used, but is less predictive, to estimate the risk of recurrent bleeding. Based upon the original data, and including endoscopic findings, rebleeding and death were rare in patients with scores of 0–2.12 A score of ≥ 5 was associated with a 10% risk of death and >25% risk of rebleeding. Other scores, based solely on clinical factors are less accurate than those that include endoscopic findings and have not been widely adopted. Three risk stratification scores have been developed for the assessment of lower gastrointestinal bleeding; however, none has garnered widespread use.13
TABLE 34.3
ROCKALL RISK SCORING SYSTEM
NONSURGICAL DIAGNOSTIC MODALITIES AND INTERVENTIONS
The most useful diagnostic studies are also the primary therapeutic approaches in the majority of cases of GI hemorrhage. Most lesions that can be visualized endoscopically can be successfully treated with endoscopic methods. Diagnostic angiography with transcatheter embolization has demonstrated effectiveness as a second-line approach and may safely allow the patient to avoid operation, particularly in high-risk patients and situations. When endoscopy does not identify the source of bleeding, other tests may be helpful. These diagnostic and therapeutic tests are discussed in this section. Algorithm 34.1 highlights a suggested approach to management of GI bleeding.
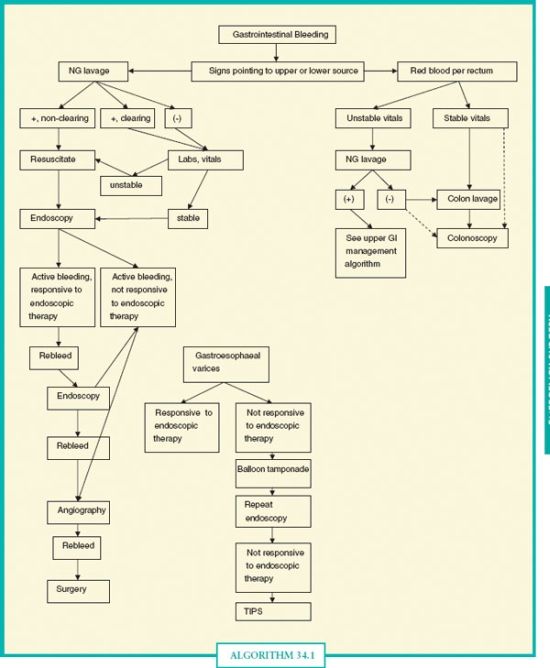
ALGORITHM 34.1 General diagnostic approach to gastrointestinal hemorrhage. The initial approach to any source of GI bleeding starts with resuscitation and stabilization of vital signs. Nasogastric lavage can help risk stratify and direct the initial endoscopic approach, but note that a negative nasogastric lavage does not rule out an UGI source of bleeding. In general, in UGI bleeding not responsive to up to two attempts at endoscopic therapy, surgery or alternative techniques (angiography) are considered. For lower gastrointestinal bleeding, colon lavage is not essential as blood can act as a cathartic, but is typically given when possible as visualization is usually much better.
Esophagogastroduodenoscopy
EGD is the procedure of choice in patients suspected of bleeding from the esophagus, stomach, or duodenum and will identify the site of bleeding in 95% of cases. There are advantages to performing EGD within 12–24 hours of presentation (“early upper endoscopy”). First, early endoscopy has been shown to reduce transfusion needs and hospital length of stay, in part, through more rapid control of bleeding.14,15 Endoscopy can identify peptic ulcers at high risk of ongoing and recurrent bleeding (active bleeding, visible vessel, adherent clot) and allow patients without high-risk features to be discharged. Endoscopy will also identify non-peptic ulcer causes with a high risk of continued hemorrhage and mortality, (i.e., gastroesophageal varices) and distinguish them from lesions with a low risk, for example, Mallory-Weiss tears. Other causes of bleeding, such as Dieulafoy lesions, while often difficult to identify are typically treated successfully with endoscopic methods.16 Effective endoscopic control of hemorrhage from a duodenal ulcer is shown in Figure 34.1. An example of perhaps the greatest benefit of upper endoscopy has been in the diagnosis and management of variceal hemorrhage secondary to portal hypertension; relegating surgery to a minimal role in the acute management. Because of its prominence in the differential diagnosis of acute upper GI hemorrhage and despite the rarity with which surgery is required to control bleeding, portal hypertension is discussed in detail in the section Four.
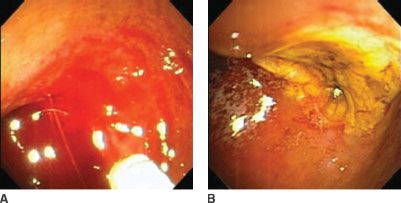
FIGURE 34.1. Endoscopic view and control of duodenal ulcer hemorrhage. A: Demonstrates intraduodenal blood with active hemorrhage evident from the upper posterior wall of the first portion of the duodenum. B: Demonstrates the same lesion after treatment. The blood has been irrigated from the duodenal lumen, and the ulcer crater is evident. Epinephrine has been injected adjacent to the bleeding vessel.
Colonoscopy
Colonoscopy is the diagnostic and sometimes the treatment modality of choice for most patients with lower GI bleeding.1 The role of early or urgent colonoscopy in the evaluation of patients with acute lower GI bleeding is less clear than is the role for endoscopy in upper GI bleeding. While best suited for patients who are actively bleeding at the time of the study, massive colonic bleeding may obscure the bleeding site and lesion, limiting the utility of colonoscopy.
Stigmata of recent hemorrhage for lower GI bleeding are similar to those of upper GI lesions and include an actively bleeding site, a non-bleeding visible vessel, and an adherent clot. Although more difficult to discover in the colon, given the large surface area and potential issues with the preparation, these findings have been associated with continued hemorrhage and the need for urgent colectomy.17 Not all studies have found colonoscopy to be accurate in the diagnosis of lower GI bleeding. Nevertheless, it is valuable in many cases and should be part of the early evaluation of patients with blood per rectum.
Diverticular bleeding is the most common source of lower GI hemorrhage in most series. However, indirect findings are often the only clue that a diverticulum is the source. Active bleeding is rarely seen, as are visible vessels. One or more diverticula may contain clot, but this is not a reliable indicator of the bleeding site. In some cases, massive bleeding caused by colonic diverticula can limit the diagnostic usefulness of colonoscopy.
Colonic angiodysplasia has a characteristic appearance and colonoscopy has been reported to have a sensitivity of 80% in identification. Angiodysplasia can be subtle and in some cases difficult to discern from subtle erosions or from traumatic or endoscopic suction artifacts. In addition, after appreciable bleeding and hypovolemia, the shunting of blood flow away from the intestinal mucosa may obscure these lesions. In cases of active bleeding from both diverticula and angiodysplasia, colonoscopy is often effective therapeutically. In diverticular bleeding, hemoclips are typically used with or without submucosal epinephrine injection; prior to the advent of hemoclips, bipolar cautery was used. In angiodysplasia, argon plasma coagulation is used as an alternative to cautery. The bipolar cautery probe can stick to the mucosa, which can complicate treatment.
Visceral Angiography and Transcatheter Treatment
Similar to endoscopy, angiographic methods can be a tool in both the diagnosis and management of upper and lower GI hemorrhage. However, its role is less established and may vary depending upon individual and institutional experience. As a diagnostic study, visceral angiography can be useful in patients with upper or lower GI bleeding in whom endoscopy has failed to identify the site of ongoing, rapid hemorrhage. This situation is particularly likely in the case of massive lower GI bleeding where colonoscopic visualization can be hampered by large amounts of blood. However, given the intermittent nature of most cases of GI bleeding, angiography is often negative, despite considerable blood loss. Unfortunately, there are no clinical features (e.g., shock, large amount or bright red appearance of blood) that correlate with positive angiographic findings.
Certain lesions often have characteristic angiographic findings that can assist in the diagnosis and guide treatment, whether angiographic, endoscopic, or surgical. Characteristic angiographic findings of angiodysplasia include a densely opacified and slowly emptying, dilated, tortuous vein (90% of patients), a vascular tuft (66%–75% of patients), and an early filling vein.
Transcatheter therapy, based upon angiographic findings, is an important treatment option, particularly in many cases of the most common causes of acute colonic bleeding. Bleeding from a variety of causes can be effectively and safely treated with angioembolization. Transcatheter therapies generally involve super-selective injection of one or a combination of materials (micro-coils, gelfoam, and vasopressin).18 Generally, series have been small but have provided important guidance. First, technical success is common (often >90%), but clinical success is much lower (50%–65%), with most failure evident as recurrent bleeding. Ischemia is another important complication, but is relatively uncommon (< 5%). It appears that clinical success is most likely for diverticular hemorrhage in comparison to other colonic sources such as angiodysplasia and neoplasms.19 Successful angiographic treatment of small intestinal sources is less common than for colonic hemorrhage.
In the case of hemorrhage from peptic ulcer disease, angiographic embolization can be used after failure of endoscopic treatment as an alternative to surgery but is generally limited to cases where the risks of surgery are prohibitive. The presence of chronic or acute comorbidities, such as morbid obesity and acute myocardial infarction should be weighed in the decision between attempts at angiographic control or surgical treatment. Angiography in peptic ulcer disease has a high initial technical success rate, but recurrent hemorrhage occurs in close to 50% of patients. Due to the rich collateral circulation of the stomach and duodenum, ischemic complications are less likely than when embolization is used for bleeding from the colon or small intestine. Successful embolization may be less likely than for mid and lower GI sources, also because of the rich collateral circulation. Therefore, while useful in select patients, it should not be considered a routine treatment option for bleeding peptic ulcers.
Radionuclide Scans
Scintigraphy using 99mtechnetium (Tc)-labeled RBCs has been used to aid in the identification and attempted localization of lower GI bleeding. It has been shown to detect bleeding occurring at rates lower than that detected by angiography but lacks the spatial resolution and diagnostic precision of angiography and endoscopy. It may be useful in the detection of intermittently bleeding lesions or those with very low rates of hemorrhage. In the past, scintigraphy was often used in cases of obscure bleeding. However, in the case of rapid bleeding, angiography may be the better option when upper and lower endoscopy have not been diagnostic. The choice between angiography and scintigraphy has typically been guided by estimates of the rate and nature of bleeding. Angiography is considered able to detect bleeding rates of >0.5–1 mL/min (30–60 mL/h) and scintigraphy is able to detect slower (>0.1 mL/min [6 mL/h]) or intermittent bleeding. However, the clinical use of these thresholds is questionable and generally they are not relevant in the determination of the appropriate test. Active hemorrhage can be detected, localized and potentially treated with angiography. Therefore, the use of scintigraphy is limited and has typically been replaced by other studies.
One area where radionuclide scanning has a clear role is in the diagnosis of Meckel’s diverticulum. 99Tc-pertechnetate is secreted by ectopic gastric mucosa in Meckel’s diverticula. This study should be considered early in the evaluation of children and young adults with lower GI bleeding.
Abdominal Computed Tomography Angiography
Standard computed tomography (CT) scanning has historically not been a reliable tool in the diagnosis of GI hemorrhage. However, technological advances allow for rapid acquisition and accurate timing of contrast administration with scanning to visualize vascular anatomy, abnormalities, and contrast extravasation.20 Multi-detector CT angiography has a number of advantages over conventional angiography. CT angiography is possible in situations where conventional angiography may not be available. Movement artifact (from respiration and peristalsis) is essentially abolished with rapid acquisition times and the use of multi-planar images to remove overlying bowel loops. Cross-sectional imaging and multi-planar reconstruction facilitates accurate anatomical localization of the bleeding site and assessment of the underlying pathology.
Approaches to Obscure Gastrointestinal Hemorrhage
Obscure GI bleeding refers specifically to bleeding that persists or recurs without an obvious source after endoscopic evaluation.2,21 Repeat endoscopy when the patient is better resuscitated may detect lesions such as ulcers or vascular ectasias that were obscured by blood or vasoconstriction at the time of initial examination. Generally, radiographic evaluation of the small bowel, including angiography or radionuclide scanning has failed to determine a source of bleeding. Most cases of obscure bleeding will eventually be found in the stomach, duodenum, or colon, and only 5% are eventually localized to the small intestine. The need for surgical evaluation is rare and is now almost entirely limited to treatment as newer diagnostic techniques have allowed lesions of the small bowel to be visualized. The techniques of balloon enteroscopy and wireless capsule enteroscopy have each facilitated the diagnosis of small intestinal sources that were previously impossible to visualize directly. Algorithm 34.2 illustrates a suggested diagnostic approach to patients with obscure GI hemorrhage.
Balloon Enteroscopy
Balloon endoscopy was developed as a technique to visualize the entire small intestine, in which the bowel is held apart by a balloon attached to the distal end of a soft overtube, through which a long enteroscope is passed. The technique has been reported to be useful for not only diagnosis but also endoscopic therapy. Single- and double-balloon techniques have been developed. Single-balloon enteroscopy is considered easier; however, double-balloon enteroscopy may have an edge in rate of complete enteroscopy and therapeutic yield. Both methods are labor intensive (require general anesthesia) and time-consuming and are performed in relatively few centers.22,23 Enteroscopy may be primarily useful in situations where standard endoscopy, CT angiography, and angiography have failed to determine the source of obscure hemorrhage and bleeding is ongoing.24,25
Wireless Capsule Endoscopy
Imaging of the small intestine is also now possible with a wireless capsule endoscope consisting of a battery, light source, imaging-capturing system, and transmitter. The typical capsule endoscope is 11 mm × 26 mm and is moved solely by peristalsis. This system captures and sends up to two images per second for about 8 hours to an ultra-high frequency band radiotelemetry unit worn by the patient. The location of the capsule is suggested by the strength of the signal. Several studies have shown high diagnostic yields using this technique and found it to be superior to push enteroscopy in patients with obscure GI bleeding.26 For an 8-hour study, approximately 57,000 images are generated. Although still considered tedious, with software advancements allowing up to four viewing frames at a time, the experienced reviewer can read an 8-hour study in <1 hour.
Intraoperative Endoscopy
Intraoperative enteroscopy using a combination of push enteroscopes per os and per rectum or via enterotomy can allow examination of the entire small bowel. While the endoscopist manipulates the scope, the surgeon manually advances the bowel over the endoscope. After the bowel is telescoped onto the endoscope, it is slowly withdrawn while the endoscopist examines the mucosal lumen and the surgeon watches the transilluminated bowel wall. With the advent of balloon enteroscopy and capsule endoscopy, interoperative endoscopy is rarely required.
SURGICAL CONSIDERATIONS AND PROCEDURES IN PATIENTS WITH GASTROINTESTINAL HEMORRHAGE
Surgery is generally reserved for patients with life-threatening hemorrhage who have failed previously discussed management options. Perhaps the greatest challenge is to identify patients requiring surgical therapy as early as possible in their course and avoid delays that may lead to increased instability and risk for complications while safely pursuing the nonsurgical approaches discussed in previous sections. Previously recommended indications for surgery, which have often reflected the severity of hemorrhage (shock, need for >4–6 units of RBCs), may no longer be appropriate. For example, in the case of bleeding from peptic ulcer disease, endoscopic retreatment after initial control of bleeding has been shown to decrease the need for surgery and results in fewer complications than surgical management, even in the presence of large-volume blood transfusion.27 In the setting of colonic diverticular disease, an association between number of units of blood transfused and risk of rebleeding has been observed; however, no clear threshold exists for units of blood transfused and the need for operation.28 In all cases, multiple factors including the source of hemorrhage, the appearance of the bleeding site at time of endoscopy, and patient comorbidities must be considered. Thus, the decision to proceed with surgery ultimately depends on clinical judgment rather than predefined, objective thresholds. In this section, the focus is on specific circumstances where operation is indicated and important aspects of specific procedures are detailed.
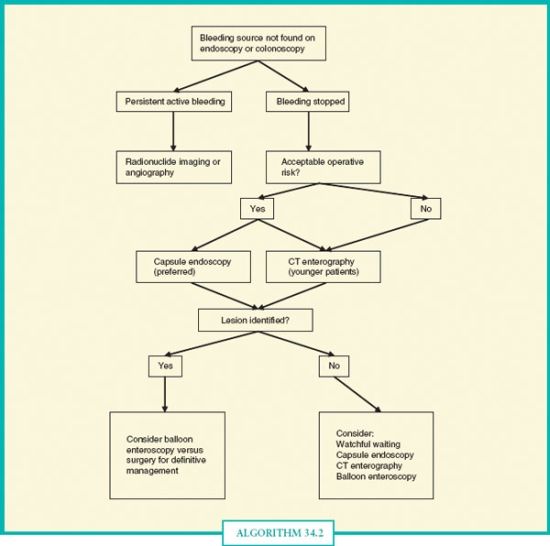
ALGORITHM 34.2 Approach to obscure gastrointestinal hemorrhage. In overt obscure bleeding that is active, radionuclide imaging and angiography may complement each other for the highest diagnostic and therapeutic yield. If bleeding has stopped, capsule endoscopy is preferred, although multiphase CT enterography should be considered in poor operative candidates (given the 1.4% rate of capsule retention), younger patients (where small bowel tumors are higher in the differential), and cases of suspected obstruction or neoplasm. Balloon enteroscopy may be considered to follow up on a positive preliminary study, or if bleeding persists and there is a high index of clinical suspicion for a small bowel lesion.
Peptic Ulcer Disease
Close collaboration between the endoscopist and surgeon is important when managing patients with high-risk peptic ulcers. Ideally, the surgeon should be available to view the endoscopy. This is particularly important in patients at high risk for needing surgical therapy (e.g., patients undergoing a repeat endoscopy for recurrent bleeding). This will facilitate surgical planning as the site of the bleeding lesion is rarely evident visually or by palpation without incision of the stomach or duodenum.
Even in cases of massive bleeding, patients can be stabilized with blood and blood product transfusion prior to induction of anesthesia. In most cases, access to the peritoneal cavity is via an upper midline incision. For proximal gastric ulcers, mobilization and retraction of the lateral segment of the left lobe of the liver is helpful for exposure and critical for the management of ulcers near the GE junction and for access to the esophagus if a truncal vagotomy is being considered.
For bleeding gastric ulcers, the operation of choice depends primarily on the location of the ulcer. Extensive gastric resections such as antrectomy, subtotal or total gastrectomy are generally not performed given that these patients are generally quite ill, at high risk of complications, and are best served by the quickest procedure that controls bleeding. Where possible, excision of the ulcer and closure of the resultant defect is ideal. This is generally applicable to ulcers of the gastric fundus, body and proximal antrum. Ulcers located near the GE junction pose a challenge, both in obtaining access and in determination of the appropriate method to control bleeding. Excision and primary closure should be done if possible without narrowing the GE junction. Alternatively, the ulcer can be oversewn after exposure through a high anterior gastrotomy.
Rarely, large ulcers on the posterior wall of the gastric antrum or body have eroded into the splenic artery or its branches, causing brisk hemorrhage. Resection is generally not possible and this circumstance is best treated by direct ligation, proximal and distal to the bleeding site, through the ulcer base. As with ulcers located near the GE junction, access to these posterior ulcers is obtained via an anterior longitudinal gastrotomy. The stomach should not be dissected from the pancreas to access the bleeding. When a gastric ulcer is left in situ , biopsy at the time of surgery is generally not necessary and instead follow-up endoscopy 6–8 weeks later is used to confirm healing or obtain tissue to exclude malignancy.
The typical duodenal ulcer that requires surgical therapy is located on the posterior–medial aspect of the first portion of the duodenum and has eroded into the gastroduodenal artery, often where it bifurcates into the superior pancreaticoduodenal and the right gastroepiploic arteries. Attention to the arterial anatomy is important to ensure successful control of bleeding. This rich anastomotic plexus often requires control of retrograde flow from these two branches in addition to suture ligation of the gastroduodenal artery. Access is obtained via a longitudinal duodenotomy and if additional exposure is needed, the incision is extended proximally across the pylorus. The ulcer and bleeding site are exposed, and active bleeding controlled with direct pressure. Ligation with 2.0 nonabsorbable suture must be done carefully as the common bile duct is typically located near and just to the right of the gastroduodenal artery. U-stitches must be placed in three quadrants to ensure control of the bleeding. Stitches are placed cephalad and caudally to the bleeding ulcer (12 o’clock and 6 o’clock) to directly control the gastroduodenal artery. A U-stitch must also be placed medially (3 o’clock) to control collateral flow from a transverse pancreatic artery. At times, the gastroduodenal artery may be additionally ligated at the superior border of the duodenum, as it dives behind the duodenum. The duodenum can then be closed longitudinally or transversely. However, if the pylorus is divided, a Heineke-Mikulicz pyloroplasty is generally indicated. The need for a vagotomy in this circumstance is debated and its reported use had been variable but definitively declining in recent years.29,30 Postoperative treatment with proton-pump inhibitors and Helicobacter pylori infection can safely prevent recurrence in lieu of vagotomy.31 Nevertheless, there may be circumstances, such as failure following H. pylori treatment, where control of gastric acid secretion by vagotomy is indicated. Vagotomy is contraindicated in the presence of hemodynamic instability, coagulopathy or acidosis. Cirrhosis, particularly when accompanied by portal hypertension, makes access to the esophagus hazardous and is a contraindication. A truncal rather than selective or highly selective vagotomy is indicated when vagotomy is performed.
Stress-Related Mucosal Disease (Gastritis and Ulceration)
Minor bleeding from gastric mucosal damage in critically ill patients is frequent, but clinically important hemorrhage is uncommon. Although surgery is rarely required, an organized management approach may reduce mortality associated with this condition.32
Coffee-ground or bloody emesis or nasogastric aspirate, coupled with hemodynamic instability, or the need for a blood transfusion mandates upper endoscopy to determine the source of bleeding. Endoscopic findings range from diffuse and superficial mucosal erosions to discrete, typically multiple, deeper ulcerations throughout the stomach.
A variety of techniques have been employed with variable success in arresting hemorrhage from stress gastropathy including endoscopic and embolization techniques and the selective catheterization of the left gastric artery with continuous infusion of vasopressin.
Surgical treatment options include gastrotomy with suture ligation of bleeding sites, hemigastrectomy, total gastrectomy, and gastric devascularization. Unfortunately, these critically ill patients poorly tolerate the more extensive procedures and lesser operations often fail to control hemorrhage. As with other causes of upper GI bleeding, endoscopic findings are important to guide surgical therapy. Regardless of the operation performed, mortality risk depends on the underlying illness, particularly in the presence of multiple organ failure. Mortality rates between 30% and 60% are commonly quoted, with as many as one-fourth of the deaths resulting from continued hemorrhage. Recurrent bleeding rates from 25% to 61% have been reported. The combination of vagotomy, hemigastrectomy, and oversewing of bleeding points has been touted as more successful in these patients. However, rebleeding rates of 11%–44% and operative mortality ranging from 33% to 63% have been associated with this procedure. More extensive operations such as near total gastrectomy or total gastrectomy are associated with significant mortality, although they successfully stop hemorrhage.
Gastroesophageal Varices
In developed countries, variceal hemorrhage is typically secondary to cirrhosis leading to portal hypertension. Generally, endoscopic therapies achieve effective immediate and longer-term control of hemorrhage and surgery is very rarely required. As a result, few surgeons have experience with emergent operative treatment of portal hypertension and variceal hemorrhage. This has further reduced the role of emergent surgery to specific and highly limited situations.
As with other sources of upper GI hemorrhage, early endoscopy is imperative for successful diagnosis and therapy in most situations. The identification of varices alone is not adequate to incriminate them as the source of the hemorrhage and it is helpful to visualize active bleeding or stigmata of recent hemorrhage. The entire esophagus, stomach, and duodenum must be adequately visualized to exclude alternate sources of hemorrhage, such as peptic ulcer disease and gastritis, which may coexist with portal hypertension and varices.
Endoscopic ligation (banding) is the most widely used modality for control of bleeding esophageal varices and is effective in up to 95% of cases. In general, a patient bleeding from esophageal varices should undergo urgent banding of the varices at the initial endoscopy.
In addition to endoscopic therapy, medical management is crucial to successful outcome. Avoiding excessive crystalloid resuscitation, which contributes to ascites formation, is important. Pharmacologic reduction in splanchnic blood flow can be helpful in reduction of variceal hemorrhage. Somatostatin or its synthetic analog, octreotide are the agents of choice and have replaced vasopressin for this indication. Meta-analyses have shown that the infusion of somatostatin is equally effective and safer than vasopressin in the pharmacologic control of variceal hemorrhage.33 In the United States, the somatostatin analog octreotide is preferentially used because it is widely available. Octreotide is typically given as an initial injection of 50 μg, followed by a 50 μg/h continuous infusion for 2–5 days. Somatostatin and its analogs are an adjunct to established methods of controlling hemorrhage, as they have never been shown to reduce mortality and their effect is to reduce transfusion requirement by about one-half unit of packed RBCs per patient.34
The use of prophylactic antibiotics in cirrhotics with GI bleeding has been shown to decrease the rate of all infections, reduce the rate of variceal rebleeding, and decrease mortality.35,36 This benefit applies to patients with and without ascites. Typically, a 3–7 day course of antibiotics, which covers gut organisms, is used, for example, ciprofloxacin 500 mg orally twice per day times seven days. Ceftriaxone is considered the preferred agent in centers with a high prevalence of quinolone-resistant organisms.37
In massively bleeding patients, balloon tamponade can be effective in temporary control of hemorrhage, and allows resuscitation, stabilization and attempts at endoscopic control when the patient is more stable. A Sengstaken-Blakemore tube consists of a single gastric lumen with proximal gastric and esophageal balloons. In a Minnesota tube, a second, proximal esophageal lumen allows the aspiration of secretions proximal to the balloons. Inflation of the gastric (and if required esophageal) balloon compresses the varices, and effectively controls hemorrhage in the majority of cases. However, hemorrhage recurs in 25%–50% of patients upon deflation, limiting this technique to a temporizing role, allowing time for resuscitation and stabilization in anticipation of definitive endoscopic treatment or as a bridge to TIPS.
These tubes can be associated with significant morbidity and mortality. Complications occur in 4%–9% of patients with the most frequent being aspiration pneumonitis. Measures to prevent pulmonary complications include endotracheal intubation before tube insertion and the placement of an esophageal tube to remove swallowed salivary secretions. Other important complications include esophageal rupture or necrosis and airway occlusion during the attempted removal of an incompletely deflated gastric balloon.
Typically, portal decompression has been recommended in the 10%–15% of cases where endoscopic and medical therapy has failed to control bleeding. Transjugular intrahepatic portosystemic shunting (TIPS) has replaced emergent surgical decompression in the acute management in these patients, but its use has typically been limited to manage recurrent hemorrhage.9 Recently, earlier use of TIPS has been advocated and appears to be associated with both better control of bleeding and increased survival at 1 year.38 Therefore, it may be reasonable to consider TIPS in patients whose bleeding has been controlled with endoscopic methods but who remain at high risk for recurrence (Child-Pugh Class C disease, or Class B with active bleeding at the time of endoscopy).
Hemorrhage from gastric varices in the context of cirrhosis and portal hypertension has been more resistant to standard endoscopic therapies.
Gastric Varices Secondary to Sinistral Portal Hypertension
This represents a unique variant of portal hypertension in which surgical therapy is primary and generally curative.39 Splenic vein thrombosis leads to portal hypertension that is generally limited to the gastrosplenic circulation and manifest as gastric varices. Chronic pancreatitis is the most common factor leading to splenic vein thrombosis, which, in turn, leads to gastric varices. The natural history of asymptomatic splenic vein thrombosis and gastric varices is benign, with few patients apparently progressing to hemorrhage. Figure 34.2 demonstrates the appearance of gastric varices that have recently bled. Splenectomy is the procedure of choice in patients who have bled from gastric varices secondary to splenic vein thrombosis.
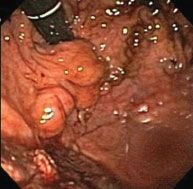
FIGURE 34.2. Endoscopic view of proximal gastric varices. This is a view of gastric fundal varices through the retroflexed gastroscope. In this case, the varices are evident as protuberances with intact, overlying mucosa, without active hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree