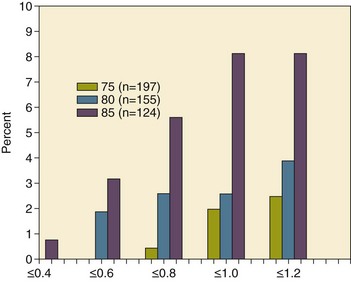
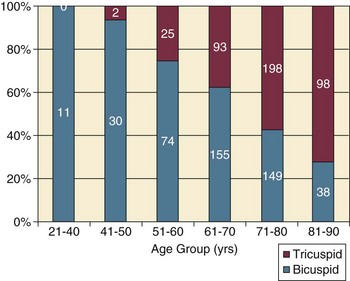
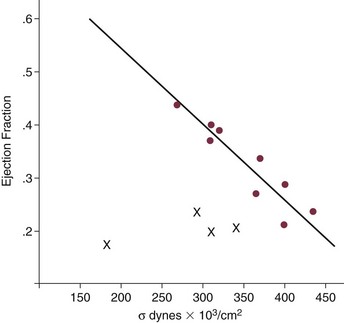
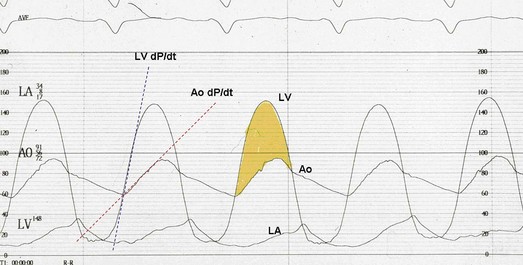
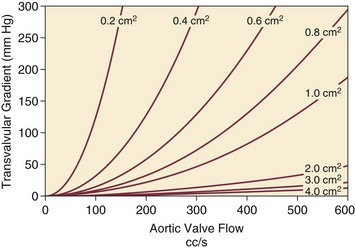
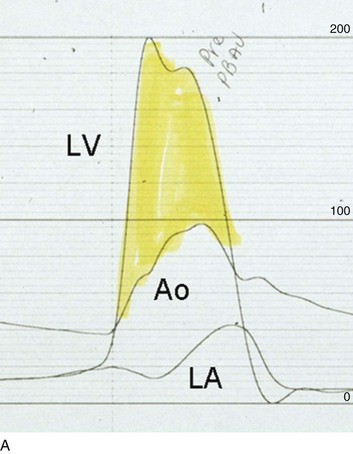
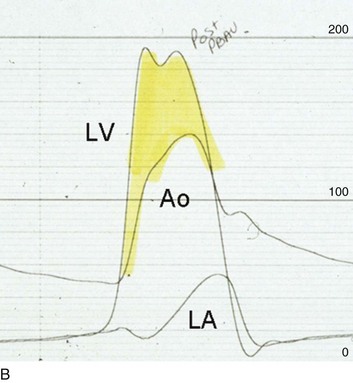
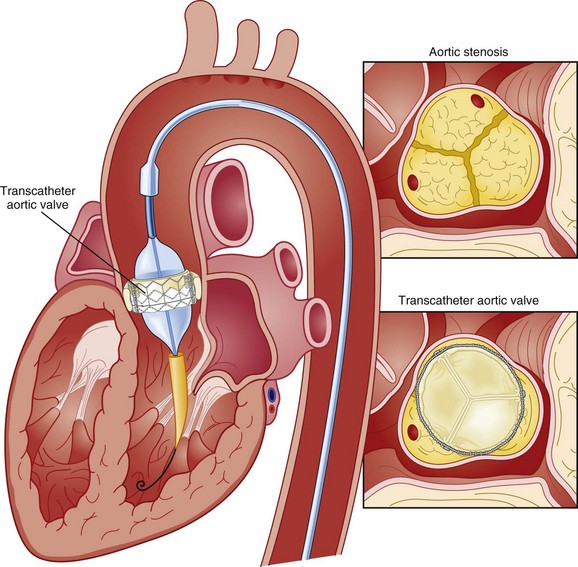
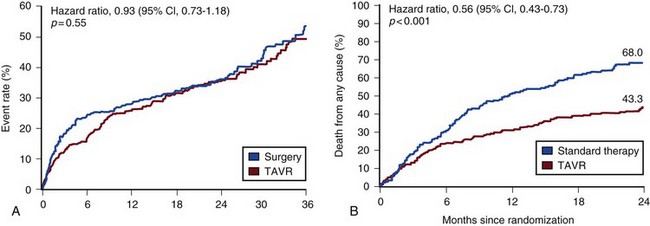
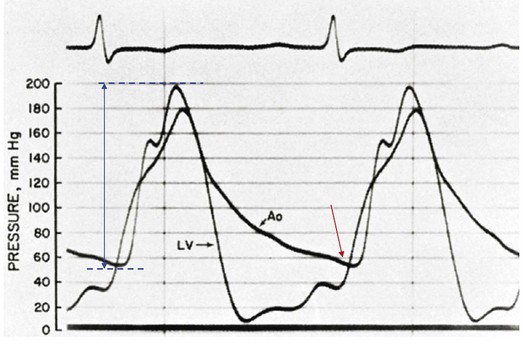
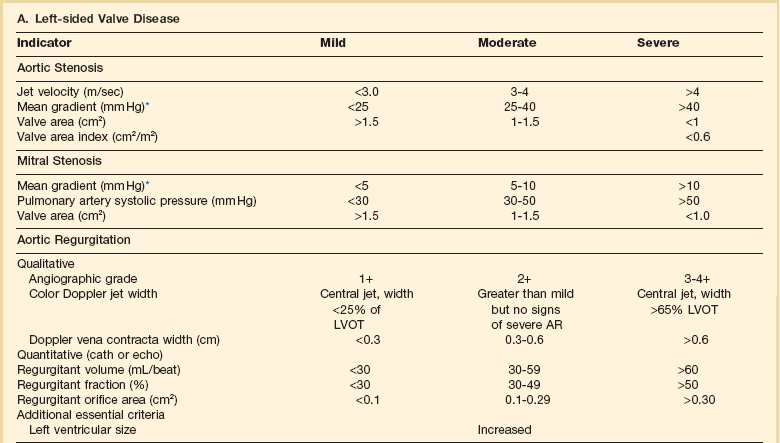
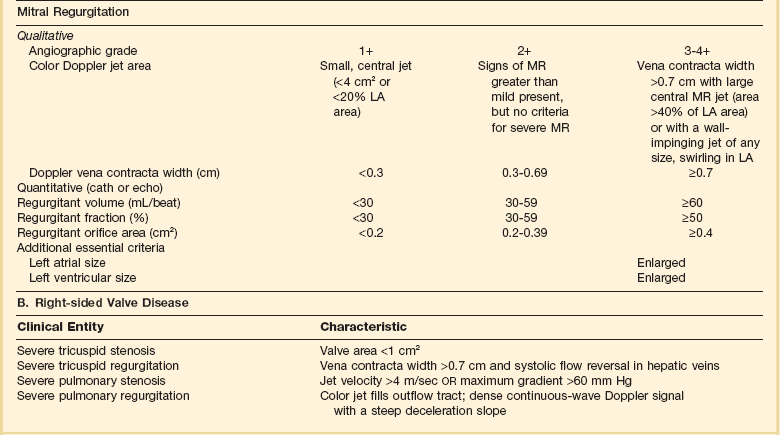
32 Rheumatic heart disease accounted for 50% or more of admissions for heart disease in the first half of the twentieth century, with congenital heart disease representing the other major indication for valve surgery through the 1960s.1 With evolving technology and an increase in average life expectancy, heart valve surgery has shifted to valve repair, as well as replacement for a variety of acquired valvulopathies. The therapeutic armamentarium, once limited to minimal supportive medical therapy, then solely to valve replacement, has expanded to a variety of surgical repair techniques; a multitude of tissue and mechanical valve options for replacement; and, most recently, an expanding set of percutaneous interventions including percutaneous valve implantation and repair. In addition, various mechanical devices for temporary hemodynamic support are important adjuncts to the management of patients with valvular heart disease in the critical care setting. Diagnosis of valvular heart disease in critical care units is challenging, with lack of a quiet environment for auscultation; comorbid conditions that affect physical findings; and stress, infection, or metabolic abnormalities that result in tachycardia and shorter intervals for evaluation of heart sounds. Patients in a low output state have softer heart sounds and murmurs as well. In addition, clinical reliance on physical examination has fallen, as has the ability to make accurate diagnosis of valve disease.2 A delay in recognizing the presence of significant valve dysfunction continues to occur frequently, and some patients who have had prior routine medical outpatient care nevertheless present with undiagnosed valvular heart disease.3 The currently accepted thresholds for mild, moderate, and severe valvular heart disease are shown in Table 32.1. Table 32.1 Classification of Severity of Valvular Heart Disease Based on ACC/AHA Guidelines *Valve gradients are flow-dependent and when used as estimates of severity of valve stenosis should be assessed with knowledge of cardiac output or forward flow across the valve. Modified from Bonow RO, Carabello BA, Chatterjee K, et al: ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists and endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol 2006;48:e1-148. With the aging of the population, aortic stenosis (AS) has moved to the forefront in frequency of valvular heart disease encountered in older populations. The prevalence is surprisingly high; in patients 85 years of age, more than 8% of a random general population survey had Doppler-derived aortic valve area estimates of 1 cm2 or less (Fig. 32.1),4 which is consistent with severe AS by the 2006 American College of Cardiology/American Heart Association (ACC/AHA) guidelines.5 Thus AS should be considered in the differential diagnosis of each elderly critical care patient with hemodynamic instability. The possibility of AS should be dismissed only after considering physical examination and electrocardiographic and, when applicable, echocardiographic and invasive findings, all of which have potential limitations in achieving accurate diagnosis as discussed subsequently.6 AS represents a continuum, from hemodynamically insignificant congenital and atherosclerotic disease to end-stage decompensation secondary to severe valvular obstruction. The congenital pathway is typically secondary to a bicuspid aortic valve, the most common congenital cardiac anomaly if mitral valve prolapse is excluded, occurring in approximately 1.5% of the population7 and originally described by Da Vinci in 1513.8 The most common acquired anomaly is typically referred to as degenerative disease, an atherosclerotic process that represents a continuum from aortic valve sclerosis, which is not associated with a significant gradient, to a densely calcified aortic valve with severe outflow obstruction. Although the cause of aortic valve stenosis in patients undergoing aortic valve replacement (AVR) has shifted to calcific tricuspid aortic valve disease from bicuspid and rheumatic disease, this remains an age-related phenomenon, with a predominance of bicuspid aortic valve disease in patients younger than age 70 (Fig. 32.2).9 Aortic valve sclerosis is an important disease entity, although usually not because of hemodynamic considerations. Defined as calcification and thickening of the aortic valve without significant outflow obstruction (gradient <20 to 25 mm Hg), it is present in nearly 30% of the population older than age 65 and nearly 50% by age 8510 and is associated with a 50% increase in 5-year cardiovascular mortality rate.11 Its incidence is as high as 15-fold greater than aortic valve stenosis; the two diagnoses can be differentiated from one another by hemodynamics and physical examination findings discussed subsequently. In keeping with the primary atherosclerotic nature of aortic sclerosis, it is associated with increasing age, male gender, hypertension, smoking, elevated low-density lipoprotein and lipoprotein(a) levels, and diabetes.10 The primary importance of aortic valve sclerosis is that it provides a window on the overall presence of vascular disease, in particular coronary artery disease.12 Thus there should be a high index of suspicion of vascular disease in any patient with aortic valve sclerosis managed in the critical care setting. Patients with aortic sclerosis do progress to aortic valve stenosis; a study of more than 2000 patients with valve thickening showed progression to severe AS in 2.5% over an average time interval of 7 years.13 The fact that so-called degenerative disease of the aortic valve is absent in nearly half of octogenarians is also important to consider because it implies that it is not only aging but other factors that result in leaflet thickening, calcification, and stenosis. In the Helsinki Aging Study from which data are reflected in Figure 32.1, additional analysis demonstrated that not only age but also hypertension and low body mass index independently predicted calcification of the aortic valve, and age and serum ionized calcium were independently associated with valvular stenosis.14 In general the process of sclerosis and then stenosis of the aortic valve appears, like atherosclerosis in general, to be an active inflammatory process, with deposition of lipoproteins, local inflammation with T lymphocyte and macrophage infiltration, fibroblast proliferation, and eventually osteoblast and bone formation.15 Similar to other vascular disease, endothelial disruption likely leads to the initial lipid deposition in the leaflet tissues. The areas of early focal plaque formation appear at the loci of greatest stress: on the aortic side of the leaflets at the flexion points. Because bicuspid valves have greater mechanical stress, the average age at presentation of patients with bicuspid aortic valve stenosis is significantly lower than in the tricuspid aortic valve stenosis that is typically seen in an elderly population.16 An important element in understanding calcific AS is lack of commissural fusion; unlike rheumatic mitral valve stenosis, in which fusion of the commissures is the primary cause of obstructive disease, lack of mobility of the aortic valve leaflets is the primary cause of obstruction in AS. Rheumatic AS is associated with commissural fusion but is now a rare finding, even in countries where rheumatic heart disease is prevalent, and is usually associated with mitral valve involvement and typically aortic insufficiency as well. More obscure causes, such as unicuspid and quadricuspid aortic valve disease, are uncommon, although presentation can be delayed to adulthood. In the case of unicuspid aortic valves there is a strong association with AS,17 whereas with quadricuspid aortic valves there is a high incidence of significant aortic insufficiency.18 Normal aortic valve area ranges from 3 to 4 cm2. Significant resistance to outflow does not occur until the valve orifice is reduced more than 50%. Based on the simple hydraulic principle of Poiseuille’s law, a 50% reduction in valve diameter results in approximately a 16-fold increase in resistance. In practice, as the resistance to outflow rises, the left ventricle is subject to pressure overload, resulting in compensatory hypertrophy. This in turn normalizes wall stress because the latter is proportional to chamber diameter times pressure divided by wall thickness (the LaPlace principle). The degree of wall thickness is variable: In patients with inadequate hypertrophic response, wall stress is inordinately high and there is early dilation and dysfunction.19 In patients with a profound hypertrophic response disproportionate to valvular resistance, wall stress actually falls below normal and ejection fraction (EF) becomes supranormal,20 a phenomenon that appears to be more common in women with AS. The EF for any degree of wall stress is predictable,19 and patients who have disproportionately poor ejection phase indices (afterload mismatch) typically manifest left ventricular (LV) dysfunction beyond the depression that would be associated with high wall stress (Fig. 32.3),21 a phenomenon described under low-gradient, low-output AS that follows. LV hypertrophy, although it reduces wall stress, has several features that may be deleterious. With progressive hypertrophy, diastolic pressures may rise as LV compliance decreases, resulting in increased LV filling pressures. In addition, the myocardial supply-demand relationship is deleteriously affected by hypertrophy in this setting. With increased wall stress and increased wall mass, myocardial oxygen demand is increased; at the same time coronary flow reserve is decreased in AS,22 and in later stages of the disease diastolic perfusion pressure is lowered. Because of abnormal flow reserve, conventional testing for ischemia in the setting of significant aortic valve stenosis has inadequate specificity to identify the presence or absence of hemodynamically significant concomitant coronary artery disease.23 Other features associated with AS that are important in the critical care setting include abnormal platelet function and decreased levels of von Willebrand factor,24 with associated bleeding risk that improves with AVR. Another associated cause of bleeding is gastrointestinal angiodysplasia associated with aortic valve stenosis,25 possibly exacerbated by the concomitant presence of von Willebrand syndrome. The progression of AS is highly variable, but with the onset of moderately severe disease, the estimated mean decrease in valve area is on the order of 0.1 cm2/year.26 Certain demographics and noninvasive findings appear to predict the rate of progression, including age older than 50 years and moderate to severe valve calcification.27 The issue of progression is particularly important in patients who are asymptomatic of the disease because AVR before it is necessary is undesirable but must be weighed against the risk of sudden death. The latter has been studied in a number of longitudinal studies and is rare if patients are followed prospectively. The patients who are most likely to require early intervention are those with peak Doppler systolic velocities greater than 4 m/second or who have a rapid increase in serial transvalvular Doppler velocity measurements.26,27 This threshold has been confirmed by other series.28 Once patients become symptomatic, with a classic triad of heart failure, angina, or syncope, hemodynamic deterioration can be rapid with significant associated mortality risk.29 The hallmarks of the physical examination relate to the contour of the aortic pressure upstroke, with a low volume and delayed carotid upstroke (pulsus parvus et tardus), a late peaking systolic ejection murmur, and a diminished or absent aortic second sound. Figure 32.4 demonstrates the hemodynamics that manifest in an abnormal carotid pulse contour; unlike the brisk upstroke of LV pressure over time (dP/dt), the aortic pressure is slow to rise and of significantly lower volume. An ejection click may be present in young patients with congenital AS, but with increasing calcification of the valve, mobility decreases to a point at which an ejection click becomes unlikely. The aortic component of the second heart sound, reflecting deceleration and closure of the aortic valve, is diminished or absent in severe AS when the thickened aortic valve leaflets are poorly mobile, have little deceleration, and drift rather than snap shut. The murmur of AS is characteristically a crescendo-decrescendo murmur that is late peaking, consistent with the timing of the peak gradient as seen in Figure 32.4, and is typically best heard over the right upper sternum and clavicle. It reflects the high-velocity systolic jet directed into the ascending aorta. A second component, described by Gallavardin as a musical component, is best heard at the left lower sternal border. The latter confounds diagnosis because the murmur is suggestive of mitral regurgitation (MR), but in its true form it is purely secondary to aortic valve stenosis. Because handgrip raises resistance to LV ejection, the murmur should decrease if it is a true Gallavardin phenomenon, whereas when it is secondary to MR, it should increase.30 Unfortunately, none of these findings has sufficiently high sensitivity or specificity for AS or for differentiating moderate from severe disease.3 Many patients with AS, particularly the elderly with stiff noncompliant vessels, will have a wide pulse pressure even with declining cardiac output and may have systolic hypertension. Hypotension may not be seen until late stages of the disease. The loudness of the murmur, which correlates to some degree with severity, is also not specific because body habitus and a variety of other factors affect the acoustics transmitted across the chest. Most importantly, in severe AS, as the cardiac output drops, the gradient generated decreases as well. In this setting the murmur may be quite soft, although sometimes still high pitched because of the high velocity across a tight constriction. A useful means of differentiating aortic sclerosis from stenosis is that in the former the systolic ejection murmur heard over the right sternum is typically midpeaking and mild to moderate in intensity, and it features a well-preserved aortic second heart sound. Although the physical findings described are variable, presence of the aortic second heart sound tends to exclude severe calcific AS.31 In contrast to the physical examination, echocardiographic techniques have progressively improved over the past several decades and availability in the critical care setting in industrialized nations is ubiquitous. The characteristic echocardiographic features of AS are decreased valve leaflet mobility, calcification in all except congenital AS in adolescents and young adults, and an augmented Doppler velocity that generally allows accurate estimation of the gradient. In congenital bicuspid AS in young adults, when commissural fusion is dominant, a characteristic doming pattern is seen, but this disappears with progressive valve calcification. The severity of calcification correlates with extent of obstruction by middle age, and typically Doppler signal velocity with peak and mean pressure gradient and valve area by continuity equation provide an accurate overall assessment. However, the gradient is highly dependent on flow across the valve, and in low-output states the gradient may result in underestimation of severity of disease; in high-output states such as in patients with augmented cardiac output caused by inotropic stimulation, endogenous high catecholamine states, and sepsis, the gradient may be disproportionately higher than the severity of stenosis would suggest. The latest ACC/AHA guidelines describe the threshold for severe AS as antegrade jet velocity greater than 4 m/second, gradient greater than 40 mm Hg, and aortic valve area less than 1 cm2, with the caveat that the gradient and jet velocity depend on the overall transvalvular flow5 (see Table 32.1). Importantly, antegrade flow across the aortic valve does not equal cardiac output in patients with confounding conditions, including aortic insufficiency; thus, for example, a patient with mild to moderate AS and moderate aortic insufficiency may have a transvalvular gradient suggestive of severe AS. Finally, although most practitioners do not index the valve area, it is important to appreciate that in patients with large body surface areas, disease that might be considered moderate in smaller patients may be functionally severe. Several caveats need to be considered before acceptance of noninvasive data in the critical care setting. Inadequate acoustic windows in some patients and difficulty in positioning patients on respirators and with multiple lines in place do limit echocardiographic imaging. Technical errors in recording and interpretation result in significant misdiagnosis: errors in recording angle, inadvertent imaging of MR jets instead of aortic outflow, assessing proximal velocity instead of the transvalvular signal, and selecting signals in the setting of arrhythmias that are not representative of mean heartbeats can all lead to skewed assessment of valve disease severity.32 Further, a number of other potential confounding variables can lead to overestimation or underestimation of the severity of AS,6,33 and valve area calculations by the continuity equation in a low-flow setting may be inaccurate.34 Because the continuity equation depends on measurement of the LV outflow track dimension, which is prone to inaccuracy, another measure of AS severity, the dimensionless index (the ratio of blood flow velocity across the LV outflow track to that across the aortic valve), has been widely adopted.35 A number of other techniques for determining the severity of AS supplement the most commonly used noninvasive tools, including three-dimensional echo, computed tomography,36 and magnetic resonance imaging (MRI).37 Overall, there has been a major a shift in the gold standard from catheter-derived to noninvasive-derived determination of AS severity, and cardiac catheterization is no longer indicated for hemodynamic assessment of aortic valve disease severity when noninvasive findings are unequivocal.5 The echocardiogram provides important additional information including severity of LV hypertrophy, LV function and size, and concomitant disease of other heart valves. The presence of regional wall motion abnormalities, in the absence of a conduction disturbance, suggests concomitant coronary artery disease. The electrocardiogram (ECG) in AS is typically abnormal and frequently features LV hypertrophy, with ST-segment abnormalities in the lateral leads typically described as a strain pattern.38 Although occasionally mistaken for anterior ischemia or infarction, with loss of R voltage across the precordium and occasionally with narrow QS complexes, the ECG is not specific for severity of AS. With increasing calcification of the perivalvular tissues, heart block is seen, typically late in the course of the disease. Cardiac catheterization is indicated in patients in whom the noninvasive data are equivocal, and coronary angiography is indicated in a range of settings identified in Box 32.1. The hemodynamics of AS are best recorded with a catheter or catheters placed simultaneously on either side of the aortic valve.6,39 Unfortunately, most laboratories record femoral artery pressure in lieu of central aortic pressure or do a catheter pullback in lieu of simultaneous pressure tracings; both techniques can result in significant misinterpretation of the severity of aortic valve disease.40 A variety of other errors in catheterization laboratory pressure measurements makes the catheter-derived valve area generally more variable than desirable in all except a few laboratories, including inherent errors in estimating rather than measuring oxygen consumption and in assuming that the Gorlin constant (originally established for a limited subset of patients41) and the valve area itself remain constant under varying loading conditions.34 In the catheterization laboratory as well, transvalvular flow may be underestimated if there is concomitant aortic insufficiency, resulting in overestimation of the severity of aortic valve disease. The critical care unit patient with a low to moderate gradient (typically <30 mm Hg) across the aortic valve in the setting of low cardiac output represents an important conundrum (and should be differentiated from the aortic sclerosis patient with low gradient not associated with depressed output). In general, the rules of hydraulics demonstrate that valve area is proportional to flow divided by the square root of the gradient.41 Thus, when the valve area is fixed, increased flow is associated with an exponential rise in gradient when valve areas are less than 1 cm2 (Fig. 32.5). When a patient in the critical care setting has depressed cardiac output and a low gradient across the aortic valve, there are two possible interpretations of these findings: either the patient has mild to moderate AS and poor LV function, or the patient has severe AS with a low EF appropriate to high wall stress (see Fig. 32.3) and secondary depression of cardiac output. In the case of the former, increasing flow results in better opening of the aortic valve, with only mild to moderate increase in gradient and an increase in the calculated valve area of 0.3 cm2 or greater.42 In the latter case, a fixed obstruction to outflow exists and increasing transvalvular flow results in a dramatic increase in gradient. In addition to increasing flow across the valve in these patients, typically with dobutamine infusion,43 the echocardiogram can be useful in several other ways44: valve calcification is suggestive of fixed outflow obstruction and, if severe, suggests that the underlying disease is severe AS rather than LV dysfunction. Preserved LV contractile reserve, with significant rise in stroke volume (>20%), peak velocity (>0.6 m/second), or mean transvalvular gradient (>10 mm Hg) at the time of dobutamine infusion is an additional useful marker. Lack of contractile reserve has been associated with lower operative survival rate45 (6% vs. 33% in one study), although it should not be the sole parameter for the decision on whether or not to perform AVR: patients who survive may manifest significant recovery in LV function postoperatively.46 Low contractile reserve should not preclude AVR in patients who do have severe fixed obstruction because their prognosis without surgery is abysmal.47 One other variation of low-gradient low-output AS involves patients with small hypertrophied ventricles and preserved EF. This subset, sometimes called high valvuloarterial impedance AS, has low gradient because of low stroke volume; the burgeoning evidence base does show improved results with AVR.48 Despite the caveats, vasodilator therapy, including nitroprusside, has been used in the critical care setting in patients with severe LV dysfunction and severe AS.49 In a select group of patients not dependent on inotropes, cardiac output rose significantly and there was overall hemodynamic improvement. Similarly, intra-aortic balloon pumping has been used, although there are only isolated case reports of efficacy.50,51 As would be expected, both nitroprusside and intra-aortic balloon pumping result in afterload reduction and some increase in transvalvular flow along with some increase in aortic valve gradient. Lipid-lowering therapy and converting enzyme inhibitors have been the subject of substantial investigation,52 although their role is in the chronic setting rather than during critical care. Two prospective randomized trials failed to show clear benefits of a statin53,54 in slowing disease progression. Some preliminary evidence suggests potential benefits of angiotensin-converting enzyme (ACE) inhibitors in decreasing progression of aortic valve calcification,55,56 but the available clinical data do not show slowing of AS progression.57 Both lipid-lowering and converting enzyme inhibitors need to be tested in larger populations earlier in the course of aortic valve disease. The acute hemodynamic results (Fig. 32.6) are poor compared with those of AVR, with a 50% reduction in gradient and an increase in aortic valve area of only 0.2 to 0.3 cm2.58 The in-hospital mortality rate was close to 10% in the National Heart, Lung and Blood Institute registry59 and is substantially higher in patients with hemodynamic decompensation and multiorgan failure. Most valves have restenosed within a few months, with a recent series showing an 87% mortality rate after a median period of less than 7 months,60 and no benefit for long-term outcomes has been shown. Indeed, the overall clinical course is not influenced by balloon valvotomy.61 Nevertheless, it can serve as a bridge to transcatheter AVR or surgical AVR in select patients with multiple comorbid conditions and is an alternative but low efficacy measure for patients who are not candidates for AVR. It is not an alternative to TAVR or surgery for patients who can undergo valve replacement, even for most patients at relatively high risk for the latter. Several settings in which balloon aortic valvuloplasty was previously considered to have a role are no longer included as indications in the 2006 ACC/AHA guidelines.5 These settings include preoperative balloon dilation in patients undergoing noncardiac surgery62; in general all but decompensated patients can withstand general anesthesia as long as careful hemodynamic monitoring and anesthesia management are performed.63 Patients who require urgent noncardiac surgery in the setting of severe symptomatic AS do have a significant perioperative risk,64 and preoperative AVR should be considered if at all possible. Balloon aortic valvuloplasty has also been proposed as a tool to differentiate myocardial dysfunction from severe AS with secondary LV dysfunction in low-gradient, low-output AS.65 The latter patients typically demonstrated substantial improvement in stroke volume and EF after balloon dilation, but dobutamine stress testing is a far safer, less morbid screening tool. Transcatheter aortic valve replacement (TAVR), a technology developed in the past decade, has revolutionized the treatment of AS. The procedure is of increasing importance in the critical care setting. Tissue valves are sewn to either balloon expandable or self-expanding stents, which are crimped onto a catheter and advanced across the stenotic native valve. Both balloon expandable (Fig. 32.7) and self-expanding technologies are widely available outside the United States. The landmark PARTNER (Placement of AoRtic TraNscathetER valves) study had two cohorts: PARTNER A enrolled high-risk but operable patients while PARTNER B enrolled patients deemed inoperable. PARTNER A randomized patients to surgical aortic valve replacement (SAVR) versus two types of TAVRs: transfemoral if the iliac and femoral vasculature was suitable for accommodating the large catheter shafts or transapical if not.66 The results of TAVR and SAVR were similar, including mortality rate, stroke, and quality of life with published follow-up through 2 years; vascular complications were higher with TAVR and major bleeding and AF were higher with SAVR66 (Fig. 32.8A). PARTNER B randomized patients with suitable vasculature to transfemoral TAVR versus medical therapy.67 Patients who received only medical therapy (with or without balloon valvuloplasty) had a 51% 1-year mortality rate compared to those inoperable patients randomized to TAVR who had a 31% 1-year mortality rate, an approximately 40% relative reduction (Fig. 32.8B). Thus, TAVR appears to be among the most dramatically successful of all modern cardiac interventions. However, it is noteworthy that the residual mortality rate in the inoperable TAVR patients remains very high, reflecting the severe comorbid conditions in patients who met inoperability criteria. There was also an initially higher stroke risk in the TAVR patients.67 The risk of stroke in follow-up appears to be largely related to patient comorbid conditions that increase stroke risk, rather than the type of valve replacement.68 The dramatic mortality risk benefit with TAVR in inoperable patients has been shown to continue through 2 years of follow-up; because the stroke risk is higher in the surviving medically treated population, the early excess stroke risk associated with TAVR largely resolves by 24 months.69 Outside the United States, TAVR has been performed in some 70,000 patients; the Food and Drug Administration initially approved TAVR for inoperable patients based on the results of PARTNER B and subsequently approved the high-risk population treated in PARTNER A. A variety of investigational uses of the TAVR platform, such as valve-in-valve replacement for patients with bioprosthetic valve dysfunction (AS and aortic insufficiency) are being performed, primarily outside the United States70; the possibility that transcatheter valve-in-valve replacement will eventually supplant reoperation may change the algorithm for choice of tissue versus mechanical AVR. The management of TAVR patients after valve replacement frequently takes place in the critical care unit. The higher rate of vascular complications associated with TAVR requires careful monitoring for limb ischemia, retroperitoneal hemorrhage, and a variety of other vascular complications.71 Conduction disturbances, particularly after use of a self-expanding TAVR platform, occur in a significant percentage of cases, requiring permanent pacemaker placement.72 A number of issues, such as the use of dual antiplatelet therapy and anticoagulation after TAVR remain to be fully investigated; most operators treat with dual antiplatelet therapy for periods ranging from 1 to 6 months; dual antiplatelet therapy plus anticoagulation for patients with AF is a combination with particularly high major bleeding risk, though the evidence base is incomplete, and many clinicians use only aspirin and warfarin in this setting. AVR remains the treatment of choice for severe AS in patients who are considered to have reasonable operable risk. Class I indications are severe AS (valve area less than 1 cm2) with symptoms or, regardless of symptoms, if patients have severe AS and are undergoing coronary artery bypass, are undergoing surgery of the aorta or other heart valves, or have LV dysfunction. A long list of class II indications generally focuses on patients who do not meet class I criteria but are thought to be at increased risk of rapid disease progression or hemodynamic compromise. In addition, patients with moderate AS who undergo other cardiac surgery generally have simultaneous AVR. Survival after AVR is excellent if LV function is preserved, with operative mortality rate in ideal candidates as low as 1%. In these patients, age-matched survival is not significantly different from patients without AS.73 In the setting of LV dysfunction, however, postoperative life expectancy is relatively poor.74 Nevertheless, conservative therapy for severe AS remains an undesirable alternative: A recent review of 453 patients treated without intervention despite severe AS had 1-year, 5-year, and 10-year survival rates of only 62%, 32%, and 18%, respectively,75 with the worst survival in patients with renal failure, pulmonary hypertension, age older than 75 years, diminished EF, and congestive heart failure. The overall prevalence of moderate or severe aortic insufficiency (AI) in the United States, as shown in the Framingham Offspring Study, ranged from 0.3% in the fifth decade of life to 2.2% for patients aged 70 to 83.76 The frequency is age and male gender related. The diagnosis of AI is more difficult and the treatment issues in many ways more complex than for AS, with less of a clear dichotomy in the decision tree for valve replacement. AI needs to be considered in light of its acuity: Acute AI is usually associated with life-threatening comorbid conditions and the resultant acute regurgitation places great stress on the left ventricle and may itself be fatal. In contrast, chronic AI may evolve from clinically insignificant to requiring surgery over decades. Unlike AS, it is not primarily a disease of the aging process (although it does occur more frequently with increasing age) and is typically secondary to one of an extensive list of systemic or structural diseases that result in insufficiency of the aortic valve. Acute AI frequently requires urgent surgery, whereas chronic AI may be managed by watchful waiting and some limited options for medical therapy. Acute AI is generally associated with leaflet involvement by endocarditis or disruption of the aortic valve’s annular structure by dissection or trauma. Chronic AI, by contrast, is caused by congenital valve abnormalities, most importantly bicuspid aortic valve disease, or by the degenerative process described earlier associated with AS. Conditions that distort the annulus and aortic root including systemic hypertension have been considered to be important causes of secondary AI, although the association between chronic AI and systemic hypertension remains to be confirmed.76,77 Rheumatic AI, almost invariably associated with mitral valve involvement as well, remains prevalent in developing countries but is an uncommon cause of AI in industrialized nations. In general, causes of AI (Box 32.2) have been divided into those that affect the leaflets primarily and those that affect the root and annulus. The former includes bicuspid and other aortic valve abnormalities, endocarditis, rheumatic aortic valve disease, the atherosclerotic process described earlier, connective tissue disorders, antiphospholipid syndrome (Libman-Sacks endocarditis), and toxicity from anorectic drugs.78 The aortic root and annulus are affected by a variety of comorbid conditions that dilate the aortic root; Marfan and Ehlers-Danlos syndromes; osteogenesis imperfecta; chronic aortic dissection; syphilitic aortitis; connective tissue disorders; and, along with the valve leaflets, ankylosing spondylitis. In isolated acute AI, the sudden onset of regurgitation imposes a large-volume load on the left ventricle in diastole prior to an adaptive process being in place. The abrupt rise in pressure is reflected by parallel development of left atrial and pulmonary vascular hypertension, frequently resulting in pulmonary edema. Because the compliance of a previously unaffected left ventricle is not sufficient to allow adequate dilation to absorb the regurgitant volume, the ventricle operates on the steep portion of its pressure-volume curve, with inadequate stroke volume to accommodate the high regurgitant flow. The effective (net) forward stroke volume (antegrade flow minus retrograde filling) is therefore low, compensated to some degree by tachycardia, but frequently insufficient to maintain normal cardiac output. This phenomenon is exaggerated in patients with preexistent LV hypertrophy in whom the ventricle is already operating on the steep portion of its pressure-volume curve, and particularly severe decompensation is seen when the left ventricle is small and hypertrophic. Critical care settings for the latter unfortunately include many of the common scenarios for acute AI including endocarditis in the setting of AS and aortic dissection in patients with hypertension and a dilated aortic root.5 Acute severe AI results in near approximation of aortic and LV pressures at end diastole, with consequent deleterious effects on coronary perfusion of the subendocardium (Fig. 32.9). Because coronary perfusion pressure is the difference between diastolic pressure in the aortic root and subendocardial pressure in the LV cavity, the decrease in aortic diastolic pressure and rise in subendocardial pressure that are hallmarks of acute AI can result in profound subendocardial ischemia, especially in patients with preexistent hypertrophy or patients with underlying coronary artery disease. In addition, because afterload and wall stress in this setting are increased, there is a rise in myocardial oxygen demand simultaneous with a fall in supply, occasionally leading to cardiogenic shock and death. Acute AI also results in early closure of the mitral valve as LV diastolic pressure rises above left atrial pressure, a phenomenon that has potential protective benefits for the pulmonary circulation. In contrast, chronic AI features a host of adaptive processes by the left ventricle including progressive dilation, increased compliance, and hypertrophy. As with AS, hypertrophy results in lower wall stress but AI features an increase in afterload combined with progressive LV dilation and somewhat less hypertrophy, resulting in significantly higher wall stress than seen in compensated AS.79 End-diastolic dilation of the left ventricle allows for larger stroke volumes to compensate for regurgitant fractions, which may be in the range of 50% or greater. With combined pressure and volume overload, unique among the valve disorders discussed in this chapter, LV adaptations allow for maintenance of normal overall function until ventricular remodeling and hypertrophy are no longer sufficient to maintain forward stroke volume and EF. With increasing wall stress and LV dilation, LV contractility is eventually impaired, at which point filling pressures begin to rise (or rise further) and patients become symptomatic. Coronary flow reserve in AI, as with AS, is impaired80 and, combined with increased demand and the need for additional perfusion for a hypertrophic myocardium, may result in significant ischemia. The onset of symptoms may be abrupt, especially with new onset of atrial tachyarrhythmias or sudden increase in cardiac output demand such as with exertion or infection. Although acute AI represents a relative or absolute medical emergency, chronic AI may have an insidious course over decades. Some insight into the rate of progression of chronic AI is provided by a meta-analysis incorporated into the most recent ACC/AHA guidelines.5 In a review of nine admittedly heterogeneous studies incorporating nearly 600 primarily asymptomatic or mildly symptomatic patients with AI, average progression to symptoms with or without LV systolic dysfunction was 4.3% annually, and sudden death occurred in 0.2% annually.5 Although this rate of progression is modest, patients do not necessarily develop symptoms before developing LV dysfunction or sudden death.81 Age, end-systolic dimensions,82 and rate of deterioration of end-systolic dimension and EF83 are more sensitive tools for predicting outcome in chronic AI. In contrast, in the setting of symptomatic AI that does not fall in the acute severe category, an annual mortality rate of 6% to 25% has been described, depending on severity of symptoms. By the 10-year follow-up, 75% had undergone AVR or died.84 The hallmarks of acute severe AI include features consistent with congestive heart failure and low cardiac output including tachycardia, dyspnea, and signs of impaired cardiac output such as peripheral vasoconstriction. The wide pulse pressure that is a hallmark of AI may or may not be seen, in part because it is dependent on LV compliance. The classic AI murmur may not be heard because of the limited gradient between the aorta and left ventricle during diastole when LV diastolic pressures in some cases approach systemic diastolic pressures. Tachycardia and diminished effective forward flow in acute AI may offset the augmented systolic and wide pulse pressure seen in later stages of the disease, and these patients may have normal or occasionally diminished pulses, although more moderate acute AI may feature the more classic findings. Early closure of the mitral valve may result in a soft first heart sound, and distortions of leaflet anatomy may result in lack of a distinct aortic second heart sound. In some cases absence of a second heart sound in a patient presenting with cardiogenic shock may be the most prominent physical finding.78 The dramatic physical findings in chronic AI are among the most familiar to physicians and trainees. A wide pulse pressure results primarily because of increased stroke volume, which augments systolic pressure,85
Valvular Heart Disease in Critical Care
Aortic Stenosis
Pathophysiology
Aortic Valve Sclerosis
Diagnosis
Physical Examination
Noninvasive Evaluation
Cardiac Catheterization
Low-Gradient, Low-Output Aortic Stenosis
Therapy
Medical Management
Percutaneous Interventions
Balloon Valvuloplasty
Transcatheter Aortic Valve Replacement
Aortic Valve Replacement
Aortic Insufficiency
Pathophysiology
Diagnosis
Physical Examination
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Valvular Heart Disease in Critical Care