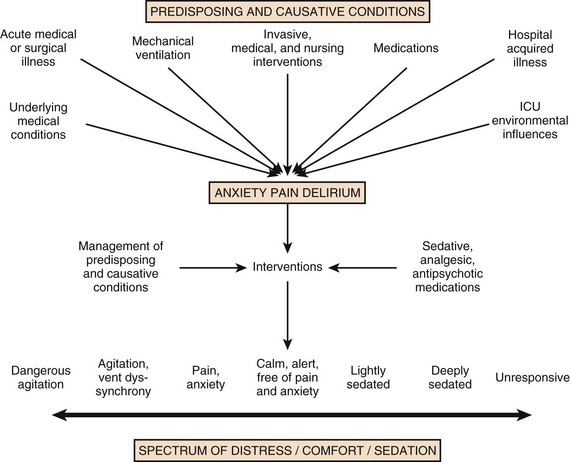
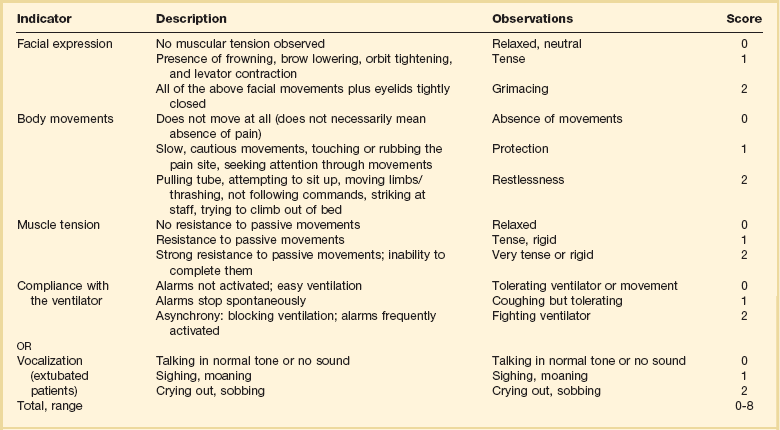
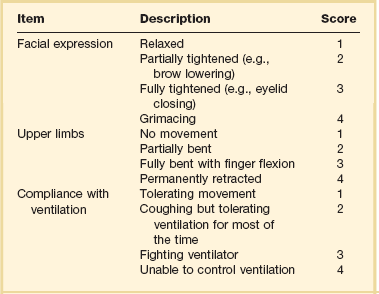
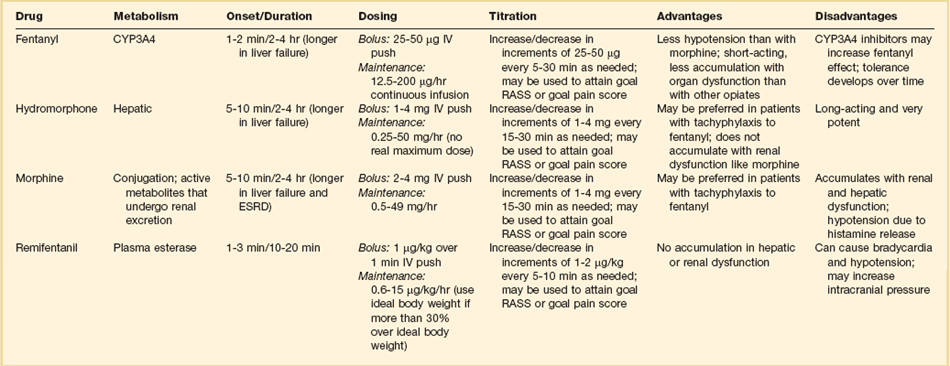
19 Critically ill patients often experience unpleasant sensations including pain, anxiety, dyspnea, and other forms of distress.1–4 This is particularly true for mechanically ventilated patients who make up a significant proportion of intensive care unit (ICU) patients. The ICU environment, and ICU care in general, is widely regarded as not ideally suited to patient comfort. For example, most patients may have surgical incisions and often have a variety of indwelling tubes and vascular catheters. Further, sleep disruption is common, sound and light levels are often excessive, and patient communication and mobility are impaired. Core principles of ICU care are to provide comfort to our patients and to relieve their suffering.5–8 Although nonpharmacologic interventions can improve patient comfort,9 clinicians have relied upon administration of analgesic and sedative medications to reduce the sensation of pain and anxiety, to control agitation, and to enhance tolerance of the ICU environment.10–12 However, clinical experience and research over the past decade have demonstrated the hazards associated with excessive or unnecessarily prolonged sedation and the importance of accurately achieving targeted analgesia and sedation.13–17 Newly revised clinical practice guidelines from the Society of Critical Care Medicine (SCCM)6 and other recent guidelines and reviews on sedation and analgesia18–23 highlight the importance of adopting a management approach that is patient-focused and strives to use the lowest effective dose of medications. The past decade has also brought a greater appreciation for the importance that development of delirium in the ICU has for long-term outcomes including downstream cognitive dysfunction.6,24–29 The topic of ICU delirium is reviewed in detail elsewhere in this textbook (Chapter 72). Finally, the use of neuromuscular blocking agents (NMBAs) to achieve therapeutic paralysis is undergoing reevaluation, particularly for optimizing management of acute respiratory distress syndrome (ARDS).30–33 The use of sedatives, analgesics, and NMBA in managing critically ill patients is reviewed in this chapter. Surveys and clinical reports indicate that the majority of ICU patients experience pain, dyspnea, or other forms of distress and receive sedative and analgesic medications.10–12,34 Some of the key terms and definitions related to patient distress and neurobehavioral abnormalities in ICU patients are presented in Box 19.1.35 Much of the discomfort is related to various stressors. In one case series, being in pain, being unable to sleep, and having tubes in the nose or mouth were the most prominent stressors reported by recovering ICU patients.4 Clearly, providing relief from these and other issues is an important goal of analgesic and sedative therapy. Consequences of suboptimal analgesia and sedative therapy can include problems related to inadequately controlled pain and anxiety or excessive sedation. Agitation, defined as “excessive motor activity associated with internal tension,” is perhaps the most widely recognized manifestation of uncontrolled anxiety or pain and is a problem because it can result in dramatic events, such as self-removal of important tubes and catheters, or even violence directed toward care providers. Overt agitation is a common occurrence among critically ill patients with rates as high as 20% of patient-shifts and nearly three quarters of patients during ICU stay.35–38 Self-removal of critical indwelling tubes and vascular catheters is the most common manifestation of overt agitation, placing the patient in peril from loss of an artificial airway and positive-pressure ventilation, abrupt cessation of infusion of critical medications such as vasopressors, and the risk and discomfort associated with reinsertion of tubes and lines.39 In prospective studies, device removal rates range widely from 22 to 157 events per 1000 patient-days40,41 and have an associated added cost.42 Documentation of patient agitation has become commonplace with the broad use of scales that address both sedation and agitation.43 In addition to device removal and care provider assault, agitation is often accompanied by an intense stress response with catecholamine surge resulting in tachycardia, hypertension, and tachypnea.44,45 Withdrawal of continuous infusion sedative and analgesic medications to promote awakening is associated with a two- to fourfold increase in circulating catecholamines46 and can produce evidence of withdrawal that peaks within 6 hours.47 Adrenergic stress response has been linked to hypercoagulability, glucose intolerance, increased catabolism, immunosuppression, sleep disturbance, and delirium. Further this can precipitate adverse events such as myocardial ischemia, increased intracranial pressure (ICP), tachyarrhythmias, and complications of uncontrolled hypertension in susceptible individuals.48 Finally, it is also worth considering that overt agitation can be a marker for extreme distress that has a cause that may be life-threatening or that could be rapidly resolved upon recognition and management.21,35,36 Endotracheal tube malposition or obstruction, severe chest pain from myocardial ischemia, and tension pneumothorax are examples of conditions for which severe agitation in the nonverbal patient may be an important clue to an urgent dangerous situation. In fact, agitation was a common clinical antecedent prior to cardiopulmonary arrest in one study.49 Although the primary effect of analgesic and sedative medications is the relief of pain, anxiety, agitation, and related problems, such relief is usually accompanied by reduced level of consciousness. Excessive and unnecessarily prolonged impaired sensorium is associated with additional complications—as a result of excessively deep sedation (i.e., pressure sores or nerve compression from reduced movement), or as a result of sedation-related delayed recovery from critical illness and mechanical ventilation with higher rates of complications and interventions related to longer ICU length of stay (LOS). Further, long-term neuropsychological consequences of ICU sedation are increasingly recognized.25 Accordingly, it is critical to take a structured approach to managing analgesia and sedation, which includes carefully examining the specific patient-focused goals of therapy, using validated tools for evaluation and monitoring, and implementing management that explicitly targets using the lowest effective dose of the best analgesic and sedative medications for the specific patient. Some important components for analgesia and sedation management gleaned from prior reviews and current clinical practice guidelines are listed in Box 19.2.6,19–23 Analgesia and sedation are most effective when they are patient-focused because each patient and circumstances have unique features that may influence the response to therapy. Some of the predisposing and precipitating conditions that can contribute to the development of anxiety, pain, and delirium are noted in Figure 19.1.19,35 For example, a variety of underlying medical conditions—such as alcohol or substance abuse,50 psychiatric disorders, and chronic pain—can influence the likelihood of developing anxiety, pain, or delirium and can affect the response to therapy.37 Further, inadvertent discontinuation of home medications such as opioids, benzodiazepines, or antipsychotic medications because of lack of awareness of their prior use is not uncommon with unscheduled ICU admissions and is an important reason to perform detailed medication reconciliation with patients and their family members. Although the notion that “ICU psychosis” is actually caused by the ICU is misleading, it is clear that excessive noise, light, and repeated patient awakening to check vital signs or perform tests at night contribute to fragmented sleep and can provoke anxiety and delirium.51–53 Acute medical illness, surgical interventions, mechanical ventilation and the interface (i.e., tracheostomy tube, endotracheal tube, oronasal mask), and nursing interventions such as suctioning and turning can contribute to pain and discomfort.3,4,54 A variety of conditions and medications have been associated with the development of delirium and agitation, as displayed in Box 19.3.35 Pain is widely experienced in the ICU setting and can result from invasive procedures, surgery, and monitoring devices, as well as more direct stimuli from injury, inflammation, and immobility.55 Treating pain is a core component of the caregivers’ responsibility to relieve suffering and offers the physiologic benefits of relieving pain-induced altered glucose control, myocardial ischemia, immune system dysfunction, hypercoagulability, ventilator asynchrony, impaired ventilation, and disrupted sleep.55 Mechanisms for these downstream effects include neurohormonal derangements, catecholamine release, and stress response.55,56 Effective treatment of pain begins with the assumption that pain is widely present and that caregivers should systematically evaluate each patient for the presence of pain and its intensity and characteristics, on a repetitive basis.6,8,9,55 Caregivers tend to underrecognize pain and often fail to preemptively treat pain.3,57 Accordingly, clinicians should err on the side of presuming pain is present—particularly when considering that it is far more effective to prevent and manage pain at an early stage than after it has become severe. Ideally, pain should be described in regard to location, duration, type, exacerbating and relieving factors, and intensity. Pain is sometimes conceptualized by subtypes, including somatic, visceral, and neuropathic, because manifestations and management can be different.55 For example, somatic pain is typically dull and aching, often localized, and responds well to opioids and nonsteroidal anti-inflammatory agents (NSAIDs). In contrast, visceral pain is often cramping and colicky and may respond to anticholinergic therapy, although the burning and shooting neuropathic pain is often best treated with antidepressant and anticonvulsant agents. Because pain is a subjective interpretation by an individual, the ability of that individual to convey the presence and magnitude of pain is of great value in guiding evaluation and management.58 Detailed verbal communication by ICU patients is often limited; however, use of simple tools such as the numerical rating scale or a series of cartoon faces ranging from smiling to crying can facilitate communication by pointing or nodding in the nonverbal but cognitively intact patient.59 A simple horizontal 0 to 10 numerical scale with enlarged font was judged to be most feasible and valid in one study of pain intensity rating scales.60 Other techniques to enhance the use of self-reporting of pain include adding descriptive words to the numerical scale, carefully explaining the tool and correct use with each encounter, providing glasses and hearing aids if necessary, allowing adequate time for instructions and patient response.58 Use of a scale that incorporates descriptive pictures, like the faces scale, can facilitate self-reporting of pain intensity regardless of potential language barriers. Many ICU patients are cognitively impaired and self-reported pain intensity is not feasible. Accordingly, clinicians must rely upon observed behaviors that have been documented to correlate well with self-reported pain or to be associated with noxious stimuli.54,57,61–63 Investigators have combined behaviors into structured assessment tools. Interestingly, much of the early work in pain assessment for noncommunicative ICU patients was in infants and young children, resulting in the development of the COMFORT scale,64 the Face, Legs, Activity, Cry and Consolability (FLACC) observation tool,65 and others. Subsequently a variety of pain assessment tools for nonverbal critically ill adults have been developed, validated, and thoroughly reviewed.58,66,67 In the 2012 SCCM guidelines,6 published pain observation tools for adults in the ICU were identified as the Critical-Care Pain Observation Tool (CPOT),68 the Behavioral Pain Scale (BPS),69 the Behavioral Pain Scale—Nonintubated (BPS-NI),70 the Non-Verbal Pain Scale (NVPS),71 the Pain Behavioral Assessment Tool (PBAT),54,72 and the Pain Assessment, Intervention, and Notation (PAIN) tool.73 Extensive psychometric testing was performed including examination of scale development, testing of reliability, and testing of validity, feasibility, and scale relevance or impact of implementation in ICU patient outcomes yielding a maximum score of 25 and weighted score of 0 to 20. The CPOT had the highest weighted score of 14.7, followed by the BPS with 12.0,6 and these two scales are recommended in the 2012 SCCM guidelines as the most valid and reliable behavioral pain scales for monitoring pain in adult medical, postoperative, and trauma ICU patients who are unable to self-report and in whom motor function is intact and behaviors are observable.6 CPOT and BPS are displayed in Tables 19.1 and 19.2. Pain is often accompanied by changes in vital signs, with tachycardia, hypertension, and tachypnea being most common. However, changes in vital signs are inconsistently associated with pain, with many confounding factors that can mask anticipated changes—such as administration of beta blockers, as well as lack of specificity with many other causes of tachycardia, hypertension, and tachypnea.74 Accordingly, 2012 SCCM guidelines recommend against the use of vital signs (or observational pain scales that include vital signs) alone for pain assessment in adult ICU patients.6 However, it is acknowledged that vital signs may be an important cue to prompt further pain assessment.6 Other potentially useful approaches include evaluating pain risk profile, use of surrogate (such as family members) report,75 and performance of an analgesic trial.3,55,58 Table 19.1 Critical Care Pain Observation Tool (CPOT) From Gelinas C, Fillion L, Puntillo KA, et al: Validation of the critical-care pain observation tool in adult patients. Am J Crit Care 2006;15(4):420-427; used with permission. Table 19.2 From Payen JF, Bru O, Bosson JL, et al: Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med 2001;29(12):2258-2263; used with permission. There are several important tenets for managing pain in the ICU. First, when initiating sedative treatment for mechanically ventilated ICU patients, analgesia should be the first step, followed by the addition of a sedative-anxiolytic agent. This “analgesia first” approach is suggested in the 2012 SCCM guidelines6 as well as many expert reviews.18–23,55 Second, the use of preemptive analgesia should be administered prior to the performance of interventions, such as chest tube removal, that are associated with having a high likelihood of producing pain.6 It is more effective to prevent pain or manage it at an earlier and milder stage than after pain has become severe. Successful pain management in the ICU incorporates pharmacologic and nonpharmacologic treatment.9 Parenteral opioids are the primary agents used to treat pain in the ICU setting, but use of selected nonopioid and atypical agents can enhance pain relief and may reduce adverse effects. The major parenteral opioids for continuous infusion in ICU patients—fentanyl, morphine, hydromorphone, and remifentanil (see Table 19.4)9—are generally considered to be equivalent in regard to analgesic potential as well as class-based adverse effects such as respiratory depression or reduced level of consciousness. Important differences among agents in regard to lipid solubility, volume of distribution (Vd), half-life, clearance, metabolism, and active metabolites have important effects on onset, peak, and offset of action, potency, and the influence of renal or hepatic dysfunction.9 Some of the medication features that can influence opioid selection for a particular patient are noted in Table 19.3. For example, morphine sulfate is metabolized to several active metabolites, including morphine-6-glucuronide, which is as active as the parent compound and accumulates in renal failure, resulting in prolonged effects including analgesia but also somnolence and respiratory depression.76 The rapid equilibration of fentanyl from plasma to lipid-rich brain tissue is responsible for its rapid onset of action but its high lipophilicity also contributed to large Vd and delayed offset of action after prolonged infusion. Remifentanil has a small Vd, and rapid clearance with metabolism by esterases, yielding a short half-life and rapid offset of action that is independent of renal and hepatic function.77,78 Several opioids not listed deserve mention. Meperidine has the potential for neuroexcitatory adverse effects such as seizures79 and is not recommended for ICU use.6 Methadone can slow the development of tolerance to other opioid agents when coadministered but has unpredictable pharmacokinetics and is not administered by continuous infusion.6 Methadone is also a potent precipitating agent for prolonging the rate-corrected QT interval (QTc).80,81 Although opioids are most effective for pain management in the ICU patient, these agents have a well-documented propensity to produce adverse effects (AEs), which are reviewed, along with comments for each AE, in Table 19.4.9,55 Comprehensive pain management should include awareness of the likely adverse effects and how best to avoid them and to recognize and manage them. Table 19.3 Table 19.4 Adapted from Erstad BL, Puntillo K, Gilbert HC, et al: Pain management principles in the critically ill. Chest. 2009;135(4):1075-1086; used with permission. It is often advantageous to use the lowest effective dose of an opioid analgesic, particularly when intolerable side effects are present. Additionally, there are clinical settings in which a nontraditional approach may be more effective, safer, and better tolerated. Employment of alternative or complementary approaches such as adding nonopioid or atypical analgesic agents, use of epidural or other neuraxial alternatives to parenteral opioid, and use of complementary nonpharmacologic interventions should be considered in selected cases.9 The use of nonopioid medications is best approached from the perspective of differentiating neuropathic pain for which anticonvulsants, antidepressants, and other agents play a primary role versus non-neuropathic pain for which opioids are the first line of therapy and other agents are used to reduce opioid dosage and reduce side effects. There is compelling evidence that the addition of oral gabapentin or carbamazepine to opioids provides superior pain relief for neuropathic pain in ICU patients when compared to opioids alone,82,83 and this use is strongly recommended in this setting in the 2012 SCCM guidelines.6 Pharmacotherapy for neuropathic pain has been extensively studied in randomized controlled trials (RCTs) in non-ICU patients, and medications including tricyclic antidepressants, selective serotonin receptor inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), anticonvulsants, tramadol, dextromethorphan, ketamine, topical capsaicin, lidocaine patch or gel, cannabinoids, and other agents have shown efficacy, albeit often in trials with small sample size and with associated side effects.84 Nonopioid adjuvant therapy in non-neuropathic pain can help reduce the quantity of opioids administered and potentially decrease the incidence and severity of opioid-related side effects. Such agents have been extensively studied in various non–critically ill populations—primarily postoperative patients, with efficacy demonstrated in meta-analyses of RCTs for corticosteroids, NSAIDs, acetaminophen, ketamine, and others.85–88 There is relatively limited research in ICU patients and most of the RCTs performed with ICU patients have been in the postoperative setting. Best studied in the ICU population are IV (intravenous) acetaminophen, cyclooxygenase inhibitors, and ketamine.89–91 As a result of the limited evidence base in ICU patients, the 2012 SCCM guidelines generally support this approach as a weak recommendation for the addition of nonopioids for non-neuropathic pain.6 The potential benefits as well as risks of nonopioids for ICU use are further discussed in reviews published in 2009.9,55 Although systemic administration of opioids for pain management in the ICU is typically by the IV route, regional approaches can offer advantages in selected cases.9 Options for regional anesthesia and analgesia (RAA) include continuous epidural analgesia (CEA) and anesthesia (central neuraxial techniques) and continuous peripheral nerve or plexus block. With medication delivery in close proximity to the spinal cord or nerves, these techniques can promote parenteral opioid sparing, which is associated with less respiratory depression and gastrointestinal hypomotility and conveys other benefits. However, these techniques introduce new issues related to needle and catheter placement and maintenance, as well as physiologic alterations related to local anesthetic block and epidural drug delivery. Critically ill patients are often at higher risk for complications due to coagulopathy, thrombocytopenia, sepsis, and hemodynamic instability. Peripheral nerve blocks are particularly useful for anatomically localized pain such as limb injury or operation. However, of all the various RAA techniques, the greatest experience in ICU patients is with CEA. Similar to other pain management issues, there is more published research to support the use of RAA in noncritical care settings, and most ICU studies focus on patient who are postoperative or have sustained injury. The recent evidence-based review undertaken to develop the 2012 SCCM guidelines provides the most relevant and current basis for use of RAA in ICU patients.6 Specific patient populations were examined, and only thoracic epidural anesthesia/analgesia for postoperative analgesia in patients undergoing abdominal aortic surgery received a strong recommendation as a superior alternative to IV opioids.92 Interestingly, high-level evidence was also available to compare lumbar epidural analgesia to IV opioids in the same population and no benefit was demonstrated.93 Patients with traumatic rib fractures are the other population for which benefit from RAA was demonstrated for ICU patients. Specifically, use of thoracic epidural analgesia was associated with superior pain control and reduced incidence of pneumonia—but more hypotension—compared to parenteral opioids,94,95 and its use is supported as a weak recommendation in this setting.6 The evidence was insufficient to recommend thoracic epidural analgesia for intrathoracic or nonvascular abdominal surgical procedures.6 Similarly, the lack of high-quality research prevented a recommendation regarding the use of RAA for medical ICU patients.6 Nonpharmacologic interventions such as music, relaxation, and provision of information can provide complementary pain relief and can be considered.9,55,96–98 However, although they are low cost, easy to provide, and safe, they have a relatively weak body of evidence to support their routine use, and they are not “high impact,” providing only limited opioid-sparing or pain intensity reduction capability.9,55,96 Sedative drugs have formed the cornerstone for patient comfort and tolerance of the ICU environment for decades by calming anxiety, providing amnesia, and controlling agitation. There have been changing paradigms from preferential use of moderate- to long-acting benzodiazepines5 and acceptance of prolonged deep sedation a decade ago, to patient-focused sedation with careful monitoring, analgesia first, and more recently to an emphasis on the use of nonbenzodiazepine sedative agents and using the lightest level of sedation possible.6 Management of sedation should be preceded by examining individual patient characteristics and effective pain control. It incorporates establishing the goals of sedative therapy, monitoring of the depth of sedation, selecting and administering the best agent(s) for the particular patient, and using protocolized management of sedation that includes structured elements to assure use of the lowest effective dose and lightest level of sedation. Common goals of therapy include relief of anxiety, control of frank agitation, promotion of patient-ventilator synchrony, and elimination of awareness when an NMBA is used. Monitoring of the depth of sedation is now routinely performed in most ICUs and is applied to titrate medications to a targeted level of consciousness and to avoid excessive or prolonged sedation. Monitoring the depth of sedation is most commonly performed by observing patient behaviors, often after varying levels of stimulation, using a structured sedation scale.43
Use of Sedatives, Analgesics, and Neuromuscular Blockers
PAIN Management in the Intensive Care Unit
Potential Adverse Effect
Comment
Allergy
Rare; most common with morphine
Arrhythmias
QTc prolongation with methadone
CNS depression
Accumulation of active morphine metabolite in renal failure
Constipation
Start bowel regimen early if sustained opioid administration is likely
Enteral naloxone is sometimes effective, but reverses analgesia
Cough
Most common with fentanyl and similar agents
Dry mouth
Use oral lubricants
Histamine release
Uncommon with fentanyl and similar agents
Slow infusion rate
Treat with combination of H1 and H2 agonists
Hyperalgesia
Paradoxic increase in pain sensitivity
More common with short-acting opioids
Myoclonus
Consider reduced opioid dose or switch agent; hydrate
Nausea/vomiting
Administer antiemetic until tolerance develops
Neurotoxicity
Avoid meperidine—associated with highest incidence
Can occur with morphine or hydromorphone, particularly at higher doses or in renal failure
Opioid dependency
Withdrawal symptoms can develop—usually coincide with clearance
Prevent by tapering; reinstitute agent if withdrawal occurs
Pruritus
Consider naloxone, serotonic antagonists such as ondansetron
Rigidity
Most common with fentanyl and similar agents but can occur with any agent at high dose
Serotonin syndrome
Reported with meperidine and methadone
Avoid concomitant serotonin reuptake inhibitor
Nonopioid and Atypical Agents
Regional Anesthesia and Analgesia
Complementary Techniques
Sedation Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine