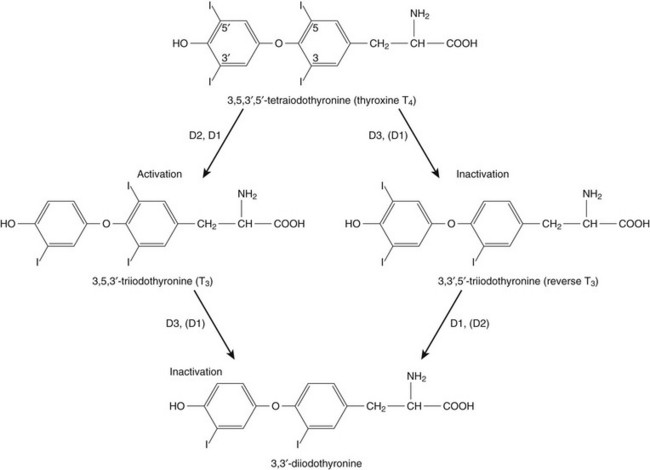
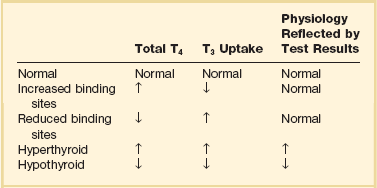
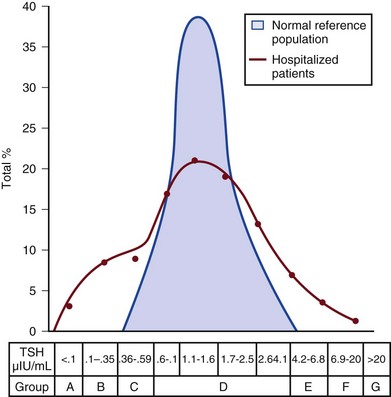
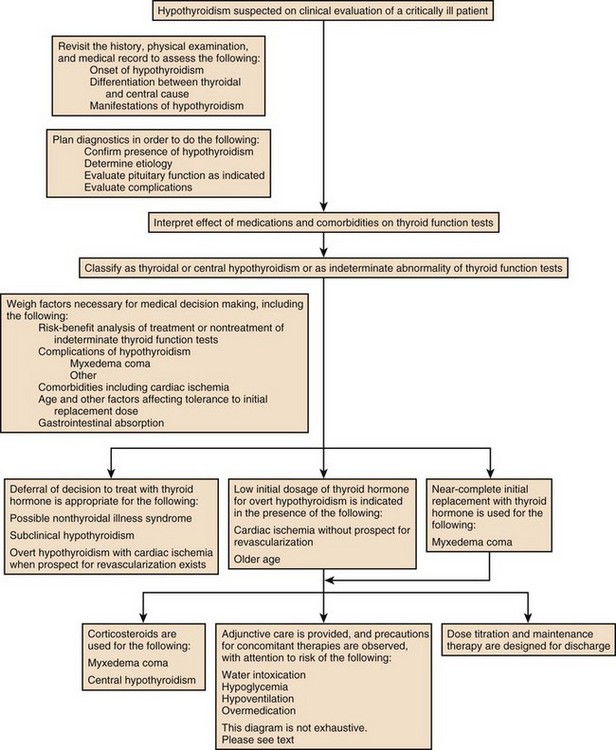
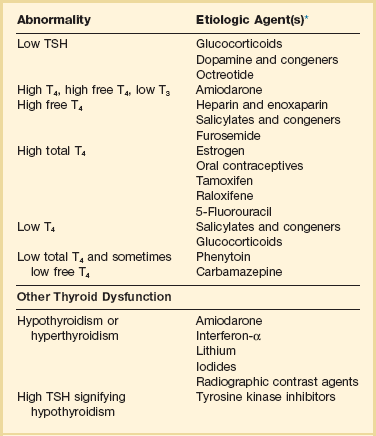
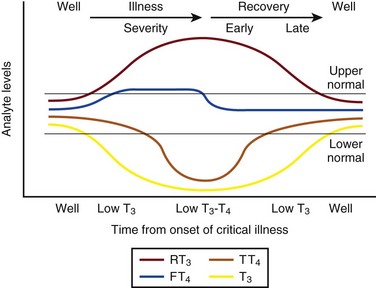
60 DIAGNOSTIC APPROACH TO THYROID DISEASE Pathogenesis of the Laboratory Findings Nonthyroidal Illness Syndrome Among Cardiac Patients Approach to Management and Therapeutic Alternatives HYPOTHYROIDISM AND MYXEDEMA COMA HYPERTHYROIDISM, THYROID STORM, THYROCARDIAC CRISIS, AND CORONARY ARTERY SPASM Thyroid hormone action is initiated by the binding of nuclear hormone receptors activated by 3,5,3′-triiodothyronine (T3) to nuclear thyroid hormone response elements, in which the hormone-receptor complex acts as a transcription factor.1 In addition, T3 exerts rapid nontranscriptional extranuclear effects that are especially important in cardiovascular physiology.2 Transporters necessary for cellular uptake of thyroid hormone recently have been recognized.3 Thyroxine (T4) acts as a prohormone for T3 and itself is only weakly interactive with thyroid hormone receptors. The compound 3,3′,5′-triiodothyronine(reverse T3) is almost devoid of metabolic activity (Fig. 60.1). Normally about 80% of circulating T3 and probably greater than 90% of circulating reverse T3 are derived from circulating T4. Peripheral production of T3 is modulated by the availability of T4, by peripheral cellular uptake of T4, and by the activities of the enzymes identified as iodothyronine selenodeiodinase 1 (D1), selenodeiodinase 2 (D2), and selenodeiodinase 3 (D3). The enzymes affecting peripheral levels of circulating thyroid hormone and tissue levels of T3 either convert T4 to T3, also removing reverse T3 by deiodination (the D1 and D2 enzymes), or deiodinate and thus inactivate T3 (the D3 enzyme) and convert T4 to reverse T3 (the D3 enzyme)4,5 (see Fig. 60.1). Circulating T4 is carried about 49% to 64% by thyroxine-binding globulin (TBG), 12% to 13% by transthyretin, and 7% to 9% by albumin. T3 is carried 80% by TBG, 9% by transthyretin, and 11% by albumin.6 A small fraction is transported by lipoproteins. About 0.03% of circulating T4 and 0.3% of T3 is free or unbound. TBG is produced by the liver, and transthyretin by the liver and choroid plexus. The approximate half-lives of circulating transport proteins are transthyretin, 2 days; TBG, 5 days; and albumin, 15 days.6 Recent research suggests that TBG allows targeted delivery of T4 to tissues, for example, at sites of inflammation.7 The unbound fraction of circulating hormone gains access to peripheral tissues and pituitary, determines metabolic status, and participates in feedback inhibition of the pituitary. In critical illness, ambiguity of thyroid function tests is commonplace. The obstacles to laboratory assessment augment the importance of record review, history, and physical examination. When overt thyroid dysfunction is present, the history and physical examination usually yield multisystemic positive findings.8 In a meta-analysis of earlier studies, the frequency of thyroid disease ascertainable by screening hospitalized patients was similar to that among outpatients, about 1% to 2%.9 In the hospital the relatively low case-finding rate and the confounding effect of nonthyroidal illness have been viewed as impediments to general screening of unselected patients, except possibly among elderly women.10,11 However, one study in which TSH and free T4 index were performed on sera drawn at the time of admission from 364 consecutive patients suggested that the rate of nonthyroidal illness syndrome (7.4%) was exceeded by the combined rates of unsuspected thyroidal failure (5.8%), subclinical hypothyroidism (6%), and hyperthyroidism (2%).12 Among critical care patients, the case-finding rate for thyroid disease during screening is not known with confidence. Clinical suspicion of thyroid disease based on patient symptoms or findings should, of course, result in testing. Among critically ill patients, the TSH assay taken alone may yield misleading results, and the utility of most commercial methods for determining free thyroid hormone levels is limited.13–17 Thyroid function test results may change on a daily basis. The best course of action is not to screen with a single test but to order a potentially useful battery of tests from the outset. Reasons for misleading TSH results, some of which apply to critically ill patients, include nonequilibrium conditions in which thyroid status has recently fluctuated, acute psychiatric illness, nonthyroidal illness syndrome, central causes of hyperthyroidism or hypothyroidism, and the effects of medication.18–20 The distribution of TSH results in nonthyroidal illness syndrome may overlap with the range seen in thyrotoxicosis. Conversely, in mild cases of subclinical thyrotoxicosis, for example, in nodular thyroid disease at the earliest stages of autonomy, TSH suppression may be minimal. The indication for measuring TSH is to support a diagnosis of primary hypothyroidism or hyperthyroidism. Although with some exceptions most free T4 assays perform well in the ambulatory setting, in critically ill patients some commercial assays for free T4 yield low results that cannot be verified by an equilibrium dialysis method.14,17 The indication for ordering a free T4 rather than total T4 assay is to assess thyroid function in the presence of suspected abnormalities of thyroid hormone transport or to clarify the significance of an abnormal total T4 result in a patient whose clinical condition appears euthyroid. A normal result of a free T4 determination together with a normal TSH is reassuring. The indication for ordering a total T4 assay is to discount hyperthyroidism in the presence of misleading free T4 elevations in euthyroid patients, to provide reassurance when considered together with an estimate of thyroid hormone binding that central hypothyroidism may be absent, or to demonstrate the presence and quantitate the severity of hypothyroidism or hyperthyroidism.13 The T3 uptake, together with a determination of total T4, is used in index methods to provide information about hormone transport and to help estimate free T4. In critically ill patients with hypothyroxinemia, the free T4 index frequently is misleading. Nevertheless, when hypothyroxinemia by a free hormone estimate is present but its interpretation is uncertain, for example, when the TSH is normal or low, it is advantageous to review a second independent assay such as the T3 resin uptake or a direct measurement of TBG to see whether qualitatively the result is consistent with reduced T4 binding sites on circulating transport proteins. Elevation of T3 uptake accompanied by low total T4 suggests reduced hormone binding to transport proteins, consistent with nonthyroidal illness syndrome. Nonelevated T3 uptake or low T3 resin uptake accompanied by hypothyroxinemia in the face of nonthyroidal illness suggests the possibility of hypothyroidism (Table 60.1). Free T3 sometimes is found to be normal among patients with nonthyroidal illness syndrome having low total T3, helping offset suspicion of secondary hypothyroidism due to organic pituitary or hypothalamic disease. Methodologic limitations in critically ill patients may result in variability of findings between assay systems. Chopra and colleagues reported that by direct equilibrium dialysis radioimmunoassay in nonthyroidal illness syndrome serum free T3 concentration was normal in approximately 83% of patients with low total T3.21–23 The indication for ordering the free T3 test is to confirm or exclude T3 toxicosis in patients suspected of having thyroid transport abnormalities, such that reliance upon total T3 might be inappropriate. Free T3 also may help identify amiodarone-induced thyrotoxicosis. For confirmation of a suspected diagnosis of thyroid dysfunction, a panel rather than a single test is recommended at the outset. The TSH should not be used as monoscreening in the hospital (Fig. 60.2). The initial “best panel” is whichever has the faster turnaround time or weekend availability, or both, at the laboratory used by the institution. The initial panel should include either a calculated free T4 index (utilizing T3 uptake or TBG together with total T4) or a free T4 estimate by any other method having rapid turnaround time, together with total T4 and TSH. These initial panels will yield unambiguous results in most cases of critical illness that are caused or complicated by preexisting clinically significant primary hypothyroidism or hyperthyroidism. Furthermore, the findings of normal TSH and normal or elevated free T4 with normal total T4 suggest euthyroidism. The isolated finding of free T4 elevation may result from sepsis or the effects of furosemide, heparin or enoxaparin. If the free T4 and TSH considered as a hormone pair appear discordant (both values high or both values low), and if the explanation or management is not straightforward, a consultation should be obtained. However, in the setting of nonthyroidal illness, if low T4, low free T4 estimate, and normal or low TSH are demonstrated during screening, to discount suspicion of secondary hypothyroidism it is sometimes helpful to order TSH, free T4 by equilibrium dialysis, and morning cortisol (Box 60.1). When unexpected thyroid function test results are reported, a medication review should be conducted. Drugs may alter the results of thyroid function tests either in vivo or in vitro without affecting thyroid function. Additionally, drugs not designed to treat hypothyroidism or hyperthyroidism may alter thyroid function.15–17 Periodic monitoring before and during long-term use should occur during use of a drug that is recognized to be a potential cause of thyroid dysfunction. Medication effects are summarized in Table 60.2. Table 60.2 Thyroid Function Test Abnormalities Induced by Medication in Euthyroid Patients T3, triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone. TSH elevation signifying hypothyroidism or TSH suppression signifying hyperthyroidism may result, or patients may remain euthyroid during treatment with any of the following: interferon-α,24,25 lithium,26,27 iodinated contrast agents,28 iodine,29 and amiodarone.30–35 During use of iodine-containing medications, hyperthyroidism of the Jod-Basedow type due to the iodine content of the drug may fail to self-resolve. However, when the mechanism of hyperthyroidism is destructive thyroiditis, as may be seen during some cases of interferon-α-induced hyperthyroidism and type 2 amiodarone-induced thyrotoxicosis, a possible sequence is hyperthyroidism followed by hypothyroidism.31,32 The prevalence of amiodarone-induced hypothyroidism has been variably reported, but it may be seen in up to 22% of treated patients from iodine-sufficient regions,35 and its occurrence may be increased in the presence of positive antithyroid antibodies.30,33 The combined findings of elevation of total and free T4 together with reduction of T3 occur in euthyroid persons receiving amiodarone; these findings taken alone, when seen together with a normal TSH, do not indicate thyroid dysfunction (Box 60.2). High TSH signifying hypothyroidism may be caused by tyrosine kinase inhibitors including sunitinib, imatinib, motesanib, and sorafenib.36–41 Nonthyroidal illness syndrome is usually recognized as a constellation of laboratory findings of uncertain clinical significance, discovered on thyroid function testing among patients having acute medical or surgical illness, the more pronounced abnormalities being associated with worse prognosis, and characterized by resolution after recovery from illness (Fig. 60.3). Although the severity of illness often makes clinical assessment difficult, patients usually appear clinically euthyroid. Laboratory findings of nonthyroidal illness syndrome may occur with psychiatric illness, starvation, congestive heart failure, acute and chronic renal failure, acquired immunodeficiency syndrome (AIDS), postoperative status, trauma, and in general with critical illness.19,42–62 Figure 60.3 Nonthyroidal illness syndrome. The time course of concentration of circulating iodothyronine levels are shown qualitatively during evolution of nonthyroidal illness syndrome. A characteristic sequence involves first the development of a low total T3, initially with normal T4 or sometimes with high free T4. Characteristically, as T3 falls, reverse T3 (RT3) rises. With duration of illness, as thyroid hormone transport protein concentrations decline, concentrations of total T3 and total T4 (TT4) both are affected, and total T4 levels fall. Later, during the greatest severity of the illness, there may be low thyroid-stimulating hormone (TSH) and low free T4, a predictor of higher mortality risk. During recovery, TSH overshoots the normal range (not shown) as T4 rises. Finally, after recovery, tests become normal. (Adapted with permission from Figure 156: In turn, Chopra had reproduced this from Nicoloff et al.) High free T4 without elevation of total T4, seen in the early stages of nonthyroidal illness, may be associated with use of certain drugs or with sepsis. Otherwise, during development of nonthyroidal illness syndrome, reduction of circulating T3 is one of the earliest and most consistently observed findings, possibly seen in over 70% of patients, depending upon the duration and severity of the illness.42,43 The decline of T3 often is accompanied by a rise of reverse T3. Patients with chronic renal failure who have low T3 do not invariably have high reverse T3 levels,44,45 and patients with HIV may have low reverse T3.52 Among hospitalized patients, intrinsic thyroid disease is less common than nonthyroidal illness syndrome.9,11,47 The prevalence of nonthyroidal illness syndrome among patients with psychiatric disease has been reported to be about 10%, often manifesting as elevated TSH or elevated T4.19,55 Abnormalities of thyroid function tests interpreted as euthyroid sick syndrome were found in 51.5% of elderly patients undergoing emergency surgery.57 Hypothyroxinemia without TSH elevation was reported in 22% of critically ill patients in one study.46 In another study of intensive care unit patients, depending upon the duration of illness and patient outcome, the findings of low total T3 approached 70% to 80%, and about half of patients had low total T4.59 In order to understand the mechanisms for observed alterations of iodothyronine molecules (T4, T3, and reverse T3) during nonthyroidal illness, under conditions of health and illness with and without specific interventions, there would need to be comparative human data on each of the following: tissue-specific functions of thyroid hormone; actual tissue levels of T4 and T3; cellular uptake of thyroid hormones; activity of each of the deiodinase enzymes in each tissue of interest, including hypothalamus and pituitary, thyroid, liver, muscle, and other tissues; tissue-specific direct and indirect mechanisms of regulation of each deiodinase enzyme, including enzyme-regulating effects of iodothyronines, cytokines, and drugs; contribution of each tissue and each of the three deiodinase enzymes to circulating levels of T3; the role of transport proteins in targeted delivery of thyroid hormone in health if any and in illness; and any adaptative or maladaptative effects of the alterations of iodothyronine availability seen in illness. Much of our present knowledge derives from experimental animal models and is complicated by possible ambiguity of experimental assay results for deiodinase enzyme activity and by the necessarily inferential nature of human data.5,7,63–96 Alterations of T3 and reverse T3 usually precede reductions of T4. Peripherally, there is reduced production of T3 by 5′-deiodination of T4, reduced removal of reverse T3 by 5′-deiodination, and increased removal of T3 by deiodination (see Fig. 60.1). The reduction in circulating T3 is believed to result from a combination of mechanisms, including changes of cellular uptake of T4, reduced activity of deiodinase enzymes that normally catalyze peripheral conversion of T4 to T3, inactivation of T3 by deiodination resulting from activity of the D3 enzyme, and possibly decline of hypothalamic TRH resulting from an enzymatically mediated increase of T3 production in the tanycytes.5 The role of cytokines in mediating some of these changes has been explored.71,76–79,81,84 Because T3 may amplify its own production at the tissue level by activating the D2 enzyme, a decline of central TSH drive to the thyroid indirectly could reduce peripheral production of T3. With acknowledgment that the finding of free T3 abnormalities may be method-dependent, studies suggest that in some but not all patients having low total T3, the results of free T3 assays may be normal.21–23 Elevations of free T4 sometimes occur in nonthyroidal illness, often accompanied by low or low normal total T4, and without TSH suppression. The isolated finding of free T4 elevation in vivo at least in some instances may represent artifact, such as could be introduced by nonselective beta blockers, furosemide, or free fatty acids (see previously, under “Assays and Imaging”). Mechanisms leading to high free T4 in the early stages of sepsis have been controversial. Circulating inhibitors of binding of T4 to transport proteins may be present such as nonesterified fatty acids.65,67–69,72 Additionally, cleavage by elastases or consumption of the TBG molecule by serine proteases may occur at sites of inflammation, possibly leading to release of free T4 to the circulation and permitting targeted delivery of T4 at sites of inflammation.82,83,85 Patients with both low T4 and low T3 generally have disease of greater severity and longer duration. Early studies of hormone kinetics in the setting of low T4 were consistent with reduced binding to vascular sites.64,66 A low serum albumin level often, but not always, permits the caregiver to predict that TBG will be low. Hypoalbuminemia is highly associated with nonthyroidal illness syndrome.57,60 Reduced concentration of transport proteins is a prevalent finding among cases of nonthyroidal illness presenting with low T4 and low T3.49 Inhibitors of hormone binding to transport proteins also may result in low total concentrations of circulating thyroid hormone. The more severely ill patients with nonthyroidal illness syndrome, usually those having low T4 levels, as well as low T3, may have low circulating levels of TSH.51,80,81,87,88 Central production of TRH and TSH may be reduced by inflammatory mediators. In a human autopsy study, reduced TRH gene expression was demonstrated among patients with an antemortem finding of low T3.80 In animal studies, it has been suggested that central conversion of T4 to the active hormone T3 in hypothalamic tanycytes under the action of the D2 enzyme, by producing local hyperthyroidism under conditions of illness, may act as a negative feedback signal that reduces hypothalamic release of TRH and pituitary release of TSH.5,88,92,94,96 Therefore, in prolonged or severe nonthyroidal illness syndrome, a central mechanism may contribute to the findings of low T4 and low T3. Investigationally, after TNF-α administration, as during recovery following nonthyroidal illness, there may be temporary overshoot of TSH into the mildly elevated range, accompanied by rising T4.51,81 Presently it is unknown whether nonthyroidal illness syndrome is adaptive or maladaptive. In starvation, low T3 syndrome may promote protein sparing.97 Human trials have not been conducted with a sufficient number of randomized, critically ill human subjects to resolve the question of whether thyroid hormone therapy is beneficial. In a nonrandomized study of patients with sepsis, T3 was used to reduce dopamine dependence.98 Treatment with T3 for human burn injury showed no benefit.99 In a small study of critically ill patients with low T3 and low T4, therapy with T4 did not correct the low T3 or improve the prognosis,100 and T4 for nonthyroidal illness may increase mortality rate among acute renal failure patients.101 Low T3 or low T3 and T4 levels are observed in the setting of advanced heart failure, after revascularization, and after myocardial infarction, providing a rationale for therapeutic trials of treatment with thyroid hormone among cardiac patients.102–121 Among patients with severely impaired left ventricular performance, the use of intravenous T3 as an alternative to standard therapy (such as dopamine) and the compatibility or usefulness of T3 in combination with other inotropic and vasodilating regimens requires further research. Correction of any reversible ischemia should first be achieved. Use of intravenous T3 shows promise as an inotrope and vasodilator.104,111 In the setting of advanced heart failure, coronary bypass or valve surgery, correction of congenital heart lesions, or in the treatment of transplantation donors and recipients, the benefits that have been attributed to intravenous T3 therapy include improvement of cardiac index with reduction of systemic vascular resistance,104,111 a reduction in postoperative episodes of atrial fibrillation,107 reduction in estimated mortality rate among high-risk patients,109 a reduced requirement for inotropic support and mechanical devices,112,113 a lower incidence of postoperative myocardial ischemia,113 improved cardiac allograft function,102 and improved neuroendocrine profile with improved ventricular performance.121 Whether the apparently beneficial cardiac effects of T3 administration are pharmacologic effects or at least partially the effects of physiologic replacement of a true hormone deficiency are unclear. Overall, the use of T3 for cardiac indications has not gained widespread acceptance. It is unknown whether the spontaneously developing alterations of tissue exposure to thyroid hormone during nonthyroidal illness are advantageous or disadvantageous to the patient. Although there is interest in evaluating T3 therapy for nonthyroidal illness syndrome, it is expected that differences in outcome resulting from such treatment will be small and difficult to demonstrate.62,122–125 Proposed approaches have not been adequately studied for safety or efficacy in critically ill patients having nonthyroidal illness syndrome. A special niche may exist for the use of T3 in the treatment of cardiac patients who require hemodynamic support. As a precaution, it is noted that high levels of T3 have been identified as a risk factor for coronary events.126 Although future research may bring about changes in the standard of care, at the present time for nonthyroidal illness syndrome most experts recommend observation without thyroid hormone treatment, with reevaluation of thyroid function tests after recovery. The philosophy of nontreatment would imply that there is no obligation to order thyroid tests unless thyroid disease is suspected. In contrast to the high frequency of finding the laboratory manifestations of nonthyroidal illness syndrome in the intensive care unit, among outpatients the prevalence of spontaneous hypothyroidism is relatively low, found in 1% to 2% in iodine-replete communities, and more commonly found in women than in men.141–143 An age-related increase of incidence of hypothyroidism exists, and the prevalence is higher in women. The appearance of overt hypothyroidism is predicted by prior isolated TSH elevation and positive antithyroid antibodies. Hypothyroidism can be induced by iodine, amiodarone, lithium, or tyrosine kinase inhibitors. Patients with severe hypothyroidism have reduced calorigenesis and oxygen consumption. Many metabolic processes proceed at a markedly reduced rate. Glycosaminoglycan metabolism is impeded, resulting in widespread tissue deposition of hyaluronan (hyaluronic acid). A contributory factor in the production of generalized edema is transcapillary albumin escape.144 Slowing of the metabolism of lipoproteins results in secondary hyperlipidemia. Hypercholesterolemia is common. Hypometabolism affects conversion of carotene to vitamin A and the rate of removal of vitamin K. Reduced ventilatory responses to hypoxia and hypercapnia appear to have a dominantly central mechanism, and there may be upper airway obstruction.145–150 The effusions of myxedema contain high concentrations of protein and cholesterol. Pericardial effusion is more characteristic than pericardial tamponade.151–154 Vasoconstriction with or without hypertension exists. The mechanisms of resistance to catecholamine effects are complex and controversial. There is reduced responsiveness to adrenergic stimuli but actual elevation of circulating norepinephrine concentration.155–157 Myocardial contractility, oxygen consumption, ejection time, diastolic ventricular compliance, stroke volume, heart rate, and cardiac index are reduced and systemic vascular resistance is increased.115,119,120 The left ventricular end-diastolic pressure is not necessarily elevated in myxedema and the cardiac index may increase in response to exercise.158–161 Nevetheless, when TSH is higher than 10 µIU/mL, there is an increased risk for congestive heart failure even at the stage of subclinical hypothyroidism.162–164 An increased occurrence of coronary artery disease also may exist.165–170 Hypomotility of the bowels is common. There may be coexistent iron losses as a result of menorrhagia or gastrointestinal bleeding. Malabsorption of vitamin B12 and folic acid may occur. A reduction in atrial natriuretic factor production may occur,171 as well as a reduction of the glomerular filtration rate. The kidney cannot excrete a water load effectively, but antidiuretic hormone deficiency cannot be consistently implicated when hyponatremia and defective intrarenal mechanisms are suspected.172–174 Calcium loading can result in hypercalcemia. Hyperuricemia commonly results from underexcretion of uric acid. Pituitary overproduction of prolactin but retarded responsiveness of the pituitary-adrenal axis to appropriate challenges may occur in myxedema. Hypertension may be attributable to myxedema. The thyroid is often atrophic but may be goitrous. The following characteristics often permit clinical diagnosis: a deep, husky quality of the voice; slow mode of speech; involuntary blepharoptosis; torpid expression; facial bloating; eyelid and infraorbital edema; sallowness, facial pallor; hearing loss; bradycardia, distant muffled heart tones; cool, dry, coarse skin; nonpitting edema of the supraclavicular fossae, hands, legs, and feet144; bruising; and the delayed relaxation of deep tendon reflexes. The patient may present with adynamic ileus. The TSH level of patients with untreated primary hypothyroidism can be lowered into the normal range by critical illness, but only rarely.175 Sometimes the severity of TSH elevation is blunted by the myxedema itself, with extreme hypothyroxinemia accompanied by TSH levels that are elevated but may be less than 20 µIU/mL. In most cases of advanced hypothyroidism the TSH will be above the normal reference range and the T4 and free T4 will be low. Therefore, in general, during nonthyroidal illness the laboratory diagnosis of coexistent primary hypothyroidism is straightforward (see Fig. 60.2). Milder cases of hypothyroidism potentially can be confused with the recovery phase of nonthyroidal illness syndrome, when transitory TSH elevation commonly occurs. The patient should be examined for goiter. The finding of subnormal free T4, positive antithyroid peroxidase antibodies, or TSH above 20 µIU/mL sometimes signifies intrinsic thyroid disease.18,51 Outpatient reassessment should occur. In critically ill patients the diagnosis of secondary hypothyroidism is not straightforward. When a low free T4 level by equilibrium dialysis and low TSH level are present, the question arises of whether the findings signify central hypothyroidism for any reason other than nonthyroidal illness syndrome. The most pressing immediate need would be to recognize and treat cortisol deficiency or pituitary mass effect. History of prior reproductive dysfunction, examination of cranial nerve function and mental status, and measurements of cortisol and other pituitary and target gland hormones, including follicle-stimulating hormone (FSH) and luteinizing hormone (LH) among postmenopausal women, may suggest preexisting pituitary dysfunction with or without tumor or the new occurrence of pituitary apoplexy or may help provide evidence of intactness of pituitary function. However, some critically ill patients lacking intrinsic hypothalamic or pituitary disease may have features of “eugonadal sick” syndrome as well as nonthyroidal illness syndrome. Measurement of cortisol or free cortisol may be of special value.54 The diagnosis of pituitary tumor or apoplexy is confirmed by pituitary magnetic resonance imaging (MRI) or CT scanning. Replacement therapy for hypothyroidism can be provided as levothyroxine (thyroxine, T4) or liothyronine (triiodothyronine, T3), each of which is available for oral or intravenous administration, but T4 is the preferred hormone for the treatment of ambulatory patients and most hospitalized patients.176–191 Normally, production of T3 from T4 occurs rapidly. Because of the prolonged half-life of T4, after each daily dose or after short-term interruption of chronic T4 therapy there are stable blood levels of T4 and T3.178,179 An ambulatory hypothyroid patient, when treated with T4 in dosage sufficient to maintain euthyroidism (normal TSH), often has normal T3 but blood levels of T4 slightly above the mean for a euthyroid patient.183 A patient whose T4 dose requirement was established before hospitalization generally should be maintained on the same dose, if it can be given orally. The oral absorption of T4 is impeded by intestinal disease or concomitant administration of iron, sucralfate, cholestyramine, colestipol, calcium, and other drugs.185 Enteric administration of T4 should be separated from these drugs by at least 2 to 4 hours. When T4 therapy is given for overt hypothyroidism, whole body oxygen consumption and myocardial work load increase. If coronary artery disease is present, the myocardial demand for increased oxygen consumption may not be met. The risks of angina, arrhythmia, or myocardial infarction indicate the need that introduction of thyroid hormone treatment of older patients should be cautious and gradual, using starting doses less than full replacement, making incremental small doses until full replacement is achieved by biochemical parameters, with assessment of tolerance before each adjustment, and with willingness to aim for less than full replacement in case of poor tolerance. Despite the need for a cautious approach in patients who may have heart disease, the long-range goal is to reduce risk for dyslipidemia, heart failure, and coronary atherosclerosis.192–198 Even younger patients sometimes may experience discomfort if replacement is introduced too quickly, experiencing myopathic symptoms that may worsen at first but eventually will resolve if treatment continues, or, rarely, cardiac symptoms.198 In case of intolerance during initiation, the risk for abandonment of treatment may be reduced if a temporary T4 dose reduction is made, with intent to work back more gradually toward full replacement. An uncommon outcome during initiation of treatment in children is increased pseudotumor cerebri. To initiate therapy for overt hypothyroidism, patients with abrupt development of hypothyroidism or young patients may be started on full replacement doses of T4. To reduce the risk of discomfort during initiation, young patients with longstanding severe untreated hypothyroidism may be started at a dose of levothyroxine 0.05 mg/day. Older patients or those with coronary artery disease should start with levothyroxine 0.025 mg/day. Increments of levothyroxine 0.0125 to 0.025 mg for older patients are made at about 3-week intervals until it is estimated that the patient is close to full replacement, and then the free T4 and TSH levels are rechecked. After a dosage adjustment of T4 therapy, 6 weeks is necessary before biochemical reevaluation will reflect a steady-state condition. After upward titration of the dose, the average adult requirement for hypothyroidism is about 0.112 mg/day levothyroxine orally. For elderly patients the dose is lower than for younger patients,180,183 and for subclinical or early hypothyroidism, the dose of levothyroxine necessary to normalize the TSH may be as low as 0.05 to 0.075 mg/day. It may be stated anecdotally that atrial arrhythmias are no contraindication to providing replacement therapy for hypothyroidism.195 Development of hypothyroidism during lithium or amiodarone therapy does not require drug discontinuation but may require thyroid hormone replacement. Subclinical hypothyroidism refers to persistent TSH elevation with normal free T4 and absence of characteristic symptoms of hypothyroidism. Mild or subclinical hypothyroidism is not likely to present short-term risks to a critically ill patient. However, evidence suggests increased long-term morbidity, including heart failure, from untreated subclinical hypothyroidism.162,164 Therefore, follow-up in the ambulatory setting is appropriate to determine whether TSH elevation is persistent. The hypothyroidism associated with medication use may remit if the initiating agent is later withdrawn, so that determination of a treatment plan for hypothyroidism in part depends upon the drug and in part depends upon the severity of symptoms and duration of intended treatment with the drug held responsible for the development of hypothyroidism (Fig. 60.4). For prolonged NPO (nil per os, nothing by mouth) status or during continuous administration of substances that may impair absorption of levothyroxine or for other indications, intravenous levothyroxine may be provided daily to substitute for enteral administration, in reduced amount compared to the ambulatory daily dose, with subsequent monitoring.* The fractional gastrointestinal absorption of a tablet of levothyroxine has been reported to be about 81%, considerably higher than the earlier estimate of 48%.183 However, concomitant administration of other substances, including calcium carbonate, may diminish absorption.186 Intestinal malabsorption and, anecdotally, development of severe right-sided heart failure may result in unusually high dose requirements for oral levothyroxine therapy. More than half of patients receiving enteral levothyroxine may develop subclinical or overt hypothyroidism after 2 to 3 weeks if a previously established levothyroxine dose is maintained concomitantly with continuous enteral feedings.189 Owing to the long half-life of levothyroxine and delayed tissue response to dosage adjustments, manifestations of myxedema evolve only slowly after interruption of therapy, so that interruption of levothyroxine during short-term NPO status generally is inconsequential. The American Association of Clinical Endocrinologists and the American Thyroid Association (AACE-ATA) guideline recommends that “Patients resuming L-thyroxine therapy after interruption (less than 6 weeks) and without an intercurrent cardiac event or marked weight loss may resume their previously employed full replacement doses.”191 For patients whose oral intake will be curtailed for a prolonged interval, the usual enteral dose of levothyroxine should be reduced by 20% to 40% to arrive at a dose for intravenous therapy.176,183,188 Subclinical hypothyroidism has not been shown to increase operative risk.200 For patients with overt hypothyroidism, there is increased risk of perioperative complications such as sensitivity to analgesics and anesthesia, prolonged ventilator dependence, hypotension, water intoxication, and iatrogenic myxedema coma. Elective surgery should be deferred until euthyroidism is attained. For emergency surgery, younger patients without coronary disease should be prepared as if they already had myxedema coma, using a preoperative intravenous bolus of levothyroxine, depending on age and transport protein status, as described later (see “Myxedema Coma”), and providing hydrocortisone coverage, with other precautions as described earlier (see “Precautions in the Care of the Hypothyroid Patient”). The risk of undiagnosed coronary insufficiency has to be considered in determining the preoperative levothyroxine replacement regimen of older patients. Emergency surgery should be deferred for 24 to 48 hours after initiation of thyroid hormone treatment if possible.188,201,202 Patients who are candidates for correction of reversible myocardial ischemia generally should undergo revascularization before one attempts to treat their hypothyroidism. The risks of immediate thyroid hormone replacement before noncardiac surgery should be weighed against the probably acceptable risks of successful operation without levothyroxine pretreatment.188,194,203–212 New onset of angina, myocardial infarction, or sudden death may occur within days or weeks after initiation of treatment for hypothyroidism.192,193 Secondary hyperlipidemia and most clinical manifestations of juvenile hypothyroidism and adult myxedema are reversible after therapy, although some features, such as anemia, may require months for correction. Our present-day knowledge of myxedema coma derives from isolated case reports and small retrospective series.213–234 Historically, the low doses of thyroid hormone normally used to initiate treatment of uncomplicated hypothyroidism, when administered enterally for myxedema coma, failed to prevent fatalities. The mortality rate was probably higher than 80%. In 1964 it was demonstrated that intravenous replacement with 500 µg levothyroxine, a dose calculated to nearly replete body stores of T4, improved the rate of survival.218 Liothyronine (triiodothyronine, T3) for intravenous injection later became commercially available. Myxedema coma arising in the community can generally be divided into episodes that arise spontaneously and those that arise in connection with a precipitating illness or event. Those arising spontaneously tend to occur during the colder months of the year. Precipitating factors may include congestive heart failure, pneumonia or other infection, bleeding, administration of hypotonic fluids, sedative and analgesic drugs, or anesthesia and surgery. The particular risk of hypoventilation probably is increased by the presence of heart failure, obesity, pleural or other restrictive disease, chronic obstructive lung disease, neuromuscular disease, or exposure to drugs that reduce respiratory drive.146,147 Myxedema coma presents with a constellation of findings including physical evidence of advanced hypothyroidism, stupor, bradycardia, hypotension, hypothermia, alveolar hypoventilation, obstipation, or ileus, and sometimes water intoxication or hypoglycemia.224 Patients are often elderly. The condition if untreated progresses to fatal hypotension. In the cases of myxedema coma arising spontaneously in the community, stupor progresses over several days, and families report that the number of hours spent sleeping has gradually increased to include most of a 24-hour period. Seizures have been reported.221
Thyroid Disorders
Thyroid Physiology
Cellular Effects of Thyroid Hormone
Peripheral and Intrathyroidal Conversions of T4 and T3
Hormone Transport
Diagnostic Approach to Thyroid Disease
History, Physical Examination, and Record Review
Case Finding by Screening
Assays and Imaging
Thyroid-Stimulating Hormone
Estimates of Free Thyroxine
Total Thyroxine
T3 Uptake or Thyroxine-Binding Globulin and Calculated Free T4 Index
Free T3
“Best Panel” for Critically Ill Patients
Medication Effects
Nonthyroidal Illness Syndrome
Incidence
Pathogenesis of the Laboratory Findings
Nonthyroidal Illness Syndrome Among Cardiac Patients
Approach to Management and Therapeutic Alternatives
Hypothyroidism and Myxedema Coma
Hypothyroidism
Incidence
Pathophysiology
Clinical Manifestations
Diagnostic Approach
Approach to Management
Continuation of Established Thyroid Hormone Therapy During NPO Status
Preparation of the Patient with Untreated Hypothyroidism for Emergency Surgery or Coronary Revascularization
Prognosis
Myxedema Coma
History and Incidence
Pathogenesis
Clinical Manifestations and Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thyroid Disorders