Chapter 36 The Difficult Pediatric Airway
I Introduction
One of the most challenging aspects facing anesthesiologists is maintaining the technical skills that are necessary for the management of the difficult airway (DA). The American Society of Anesthesiologists (ASA) guidelines define a difficult airway as the clinical situation in which a conventionally trained anesthesiologist experiences difficulty with face mask ventilation of the upper airway, difficulty with endotracheal intubation, or both.1 Recent reports demonstrate how important skilled airway management is to the practice of pediatric anesthesia. Data from the ASA Pediatric Closed Claims Data Base demonstrate a greater frequency of adverse respiratory events in the pediatric population.2 In the pediatric closed claims analysis, respiratory events accounted for 43% of all adverse events, most frequently related to inadequate ventilation (20%). Esophageal intubation, airway obstruction, and difficult intubation (DI) combined accounted for 14% of the remaining adverse respiratory events. In the Pediatric Perioperative Cardiac Arrest (POCA) registry, 20% of all cardiac arrests were attributed to the respiratory system.3 Airway obstruction and DI were responsible for 27% and 13% of these events, respectively. Incidence of difficult mask ventilation in nonobese children is 2.1%. Most of the patients who experience arrests from airway obstruction or DI have an underlying disease or syndrome.
II Anatomy of the Pediatric Airway
The pediatric airway, particularly in infants, is different from the adult airway. Understanding these differences is important when managing the pediatric airway. Following is a brief review of the anatomy of the normal pediatric airway.4–7
A Larynx
The larynx is situated more cephalad at the third and fourth cervical vertebrae (C3-C4) level in the infant and migrates to the adult C5 level by 6 years.6 Because the infant’s larynx is more rostral (higher), the tongue is located closer to the palate and more easily apposes the palate. As a result, airway obstruction may occur during induction or emergence from anesthesia. A common misnomer is that the infant’s larynx is more “anterior” when it is really more “rostral” or “superior” in the neck, compared with the adult larynx. In syndromes associated with mandibular hypoplasia, such as Pierre Robin, the larynx is actually positioned more posteriorly than normal. This results in a greater acute angulation between the laryngeal inlet and the base of the tongue. In this circumstance, direct visualization of the glottis may be difficult or impossible. Because of the cephalad position of the larynx and the large occiput, the “sniffing” position does not assist in visualization of the pediatric larynx.4,7 Elevating the head only moves the larynx into a more anterior position. Infants should be positioned with the head and shoulders on a flat surface with the head in a neutral position and the neck neither flexed nor extended.5
B Epiglottis
The infant epiglottis is long, stiff, and often described as Ω or U shaped.6 It projects posteriorly above the glottis at a 45-degree angle. Because the epiglottis is more obliquely angled, visualization of the vocal cords may be difficult during direct laryngoscopy. It may be necessary to lift the tip of the epiglottis with a laryngoscope blade to visualize the vocal cords. Straight laryngoscope blades are often preferred for this reason. If the patient is not paralyzed, use of a Macintosh blade is less stimulating because it is not necessary to lift the epiglottis.
C Subglottis
The cricoid cartilage is the narrowest portion of the infant’s airway, about 5 mm in diameter, compared with the vocal cords of the adult airway.6 The infant’s larynx is funnel shaped with a narrow cricoid cartilage, whereas the adult airway is cylindrical. Tight-fitting endotracheal tubes that compress the mucosa at this level may cause edema and increase resistance to flow. Resistance to flow is inversely proportional to the radius of the lumen to the fourth power (r4). One millimeter (1 mm) of edema can reduce the cross-sectional area of the infant trachea by 75%, versus 44% in the adult trachea.
III Evaluation of the Pediatric Airway
The evaluation of the pediatric airway should begin with a history and physical examination of the head and neck. The examinations mostly involve subjective experience, and consistent evaluation criteria should improve the ability to predict the DA. Clues to a potentially difficult airway include snoring, noisy breathing, difficulty breathing with feeding or an upper respiratory tract infection, and recurrent croup. Review of previous anesthesia records should be performed if available. If a DA is encountered, documentation of events and the ability to mask-ventilate is helpful for future caregivers. A prior uneventful anesthesia does not guarantee success the next time.4,7
Knowledge of syndromes that may adversely affect the airway is crucial to the management of the difficult pediatric airway. The presence of one anomaly mandates a search for others. A common feature in patients with many of these syndromes is micrognathia. Micrognathia creates more difficulty with displacement of the tongue during direct laryngoscopy, thus increasing the chance that the glottis will be difficult to visualize.4,7 The ability to intubate often changes as the child grows. Intubation often becomes easier with syndromes associated with micrognathia (e.g., Pierre Robin) as the patient ages. In mucopolysaccharide disorders or abnormalities involving the cervical spine (e.g., Klippel-Feil syndrome), intubation may become more difficult as the child ages.5
Abnormalities of the ear or the presence of ear tags has been suggested as an indicator of DI.5 In one study, bilateral microtia was associated with an increased incidence of DI (42%, vs. 2% in unilateral microtia). Mandibular hypoplasia was associated with bilateral microtia 10 times more than with unilateral microtia (50% vs. 5%), thus allowing bilateral microtia to be used as an indirect predictor of DI.8
Physical examination must focus on the head, neck, and cervical spine. Many evaluations used to predict DA in adults have not been extrapolated to the pediatric population. Cooperation of the patient is necessary for precise evaluation. In the young or uncooperative child, appropriate evaluation is limited. Preliminary data indicate that the Mallampati classification may be an insensitive predictor of DI in the pediatric population.9 Pediatric anesthesiologists are at a disadvantage because they are anesthetizing patients with less objective airway information available. This underscores the need for a skilled approach to the difficult pediatric airway.
Evaluations should focus on the size and shape of the mandible, size of the mouth and tongue, absence or prominence of teeth, presence of loose teeth, and the neck length and range of motion. Berry10 suggests that the appropriate thyromental distance in infants is one finger breadth (1.5 cm). Lateral examinations of the head and neck may provide clues to the presence of micrognathia. Mandibular enlargement has also been identified as a risk factor for DI.
Cherubism is a childhood disease consisting of painless mandibular enlargement with or without maxillary involvement that progresses rapidly in early childhood and then regresses during puberty. In cherubism, the potential displacement space is encroached on by mandibular enlargement.11 Palpation of the soft tissue of the potential displacement area may reveal the problem.
A Diagnostic Evaluation
Magnetic resonance imaging (MRI) and computed tomography (CT) may be extremely helpful in the evaluation of airway pathology. Flexible fiberoptic endoscopy may be of benefit before intubation when visualization of vocal cords is thought to be difficult or when airway pathology is suspected. In patients with unilateral hemifacial microsomia, radiographic classification of the mandibular anatomy can help predict ease of intubation.12
Radiographic evaluation of patients with airway obstruction may be obtained in patients who present to the emergency room only if they are not in respiratory distress. Radiographs should be obtained in the upright position because obstruction may worsen in the supine position.13 In this situation, it is mandatory that a clinician skilled in airway management and capable of managing a difficult pediatric airway accompany the patient along with the appropriate equipment.
Radiographs have high sensitivity (>86%) for the diagnosis of airway foreign body, exudative tracheitis, and innominate artery compression. For laryngomalacia and tracheomalacia, radiography has much lower sensitivity (5% and 62%, respectively).13 Radiologic evaluation should not take precedence over airway control in patients with a compromised airway. Other physicians, especially the otolaryngologist, may be consulted and may support a DI.
IV Classification of the Difficult Pediatric Airway
Difficulty with ventilation, intubation, or both is the definition of a difficult airway according to the ASA DA management guidelines.1 Recognition of the DA along with the circumstances that predispose to airway problems is crucial to the safe management of the pediatric airway. Classification of the difficult pediatric airway may be made according to the anatomic location affected. Major anomalies of the head, face, mouth and tongue, nasopharynx, larynx, trachea, and neck are listed.
V Pediatric Airway Equipment
To manage a DA successfully, the appropriate equipment should be immediately available. We recommend the creation of a difficult pediatric airway cart stocked with equipment for patients ranging from premature infants to small adults. In addition, the American Academy of Pediatrics Section on Anesthesiology recommends the creation of a DA cart for all locations anesthetizing children.14 This cart should be dedicated only for use in a DA or a “cannot intubate, cannot ventilate” scenario (Box 36-1).
Box 36-1 Pediatric Difficult Airway Cart
A Face Mask
Face masks have been modified for fiberoptic intubation (FOI) in a variety of ways.15–18 Frei and colleagues16,17 described modifying a commercially available mask (Vital Signs) by drilling a hole into the lateral aspect of the mask and attaching a corrugated silicon tube. The center of the mask is fitted with a plastic ring covered by a silicon membrane. A hole 1 to 2 mm smaller than the outer diameter (OD) of the bronchoscope is punched into the membrane. This airway endoscopy mask has been used to facilitate fiberoptic bronchoscopy (FOB) in patients ranging in age from 3 days to 12 months with spontaneous ventilation and propofol sedation.15 A commercially available face mask with a ventilation side port (MERA, Senko Ika Kogyo, Tokyo) was modified and used successfully to intubate fiberoptically nine patients age 3 months to 11 years under inhalational anesthesia with continuous manual ventilation.18
B Oropharyngeal Airway
Upper airway obstruction may occur during induction of anesthesia because the infant’s tongue is large in relation to the oropharynx. Appropriately sized oropharyngeal airways are necessary for air exchange. Guedel and Berman airways are the most common airways available. By holding the airway next to the child’s face, the correct size can be estimated. If the airway is too short, obstruction may be worsened. If the airway is too long, the epiglottis or uvula may be damaged. Use of a tongue depressor to insert the oropharyngeal airway is recommended to avoid impaired lymphatic drainage of the tongue.4
C Nasopharyngeal Airway
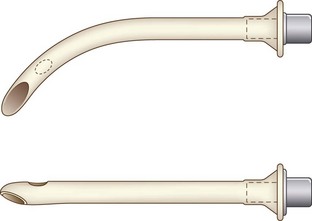
Nasopharyngeal airways are available in sizes 12 to 36 French (F) and are used with caution in pediatric patients with hypertrophied adenoids. The modified nasal trumpet was first described by Beattie, followed by its use in pediatric airway management as described by Holm-Knudsen in 2005 (Fig. 36-1).
D Endotracheal Tube
Endotracheal tubes (ETTs) in a variety of sizes (2.5-7.0 mm) should be available for the pediatric patient. Laser-resistant, nasal/oral Ring-Adair-Elywn (RAE), and wire-reinforced ETTs are available for use depending on the surgical requirement. Determination of correct ETT size is based on the patient’s age and weight. ETTs one-half size larger and smaller than the calculated size should be available (Fig. 36-2). Traditional teaching advocates the use of uncuffed ETTs in patients younger than 8 years. Pediatric ETTs with low-pressure high-volume cuffs are available for use in patients with low lung compliance or those at risk for aspiration. For cuffed ETTs, a half-size smaller tube should be used because the OD of the tube is larger with the cuff.19
Maintenance of air leak pressure at less than 25 cm H2O with or without a cuff is recommended to minimize the occurrence of postintubation croup. Use of a manometer is recommended to avoid overinflation of the cuff. Koka and associates20 cite the incidence of postintubation croup as 1%. In a prospective study of more than 5000 children, however, Litman and Keon21 found that seven patients developed croup, defined as inspiratory stridor at least 30 minutes in duration, for an incidence of 0.1%. In that study, ETTs with air leak pressures greater than 40 cm H2O were replaced with the next smaller size.21 The presence or absence of a leak depends on the level of anesthesia and the use of muscle relaxants. Many clinicians use the degree of difficulty in passing the ETT below the vocal cords as the indicator of proper fit.
In general, there are many formulas to calculate the appropriate size of ETT. Formulas for selecting an uncuffed ETT in children older than 2 years include (age + 16)/4 or (age/4) + 4. The use of cuffed ETTs in newborns and children under 8 years has been studied. In a group of 488 patients, patients were randomly allocated to receive a cuffed or an uncuffed ETT.22 The formula for the cuffed tube was (age/4) + 3. This formula was appropriate for 99% of patients. In that study, three patients in each group were treated for croup symptoms. Formulas for length of insertion of an oral ETT include length (cm) + 3 times internal diameter (mm) or length (cm) = age (years)/2 + 12.19 In the premature or newborn infant, the rule is tip-to-lip distance in cm = 6 + weight (kg).23 Whatever method is chosen, correct ETT position should be confirmed by auscultation of bilateral breath sounds (Tables 36-1 and 36-2). Also, leak should be checked to a permissible pressure.
TABLE 36-1 Suggested Endotracheal Tube Size for Infants
| Age | Size (mm ID) |
|---|---|
| Preterm (>1000 g) | 2.5 |
| Preterm (1000-2500 g) | 3.0 |
| Newborn to 6 months | 3.0-3.5 |
| 1-2 years | 4.0-4.5 |
| >2 years | (Age +16)/4 = ID |
ID, Internal diameter.
TABLE 36-2 Formula for Endotracheal Tube (ETT) Size and Depth of Insertion
| Type/Insertion | Formula |
|---|---|
| Uncuffed ETT | (Age + 16)/4 or ETT >2 years, Age/4 + 4 |
| Cuffed ETT | Age/4 + 3 |
| Length of insertion (oral) | Age (years)/2 + 12 or 3 × ID (mm) |
| Length of insertion (nasal) | 3 × ID (mm) + 2 |
ID, Internal diameter.
Double-lumen tubes are not available for use in pediatric patients younger than 6 to 8 years. The Arndt Endobronchial Blocker (Cook Critical Care, Bloomington, Ind) has been used to provide one-lung ventilation in infants.24 The 5.0-F blocker is available; the recommended ETT size is 4.5 mm. The Univent tube (Fuji Systems, Tokyo) is a single-lumen tube with an incorporated movable bronchial blocker inside.25 Pediatric sizes of the Univent tube are available: 3.5-mm internal diameter (ID) and 4.5-mm ID. The 3.5-mm Univent tube does not have a lumen for suctioning or administration of oxygen to the blocked lung. FOB is needed for placement. Further detail regarding one-lung ventilation is provided in Chapter 26.
E Endotracheal Tube Exchangers
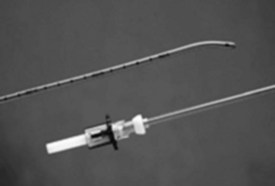
Endotracheal tube (ETT) exchangers have multiple uses; they can be used to exchange damaged ETs and provide a conduit for reintubation, if necessary. Many different types of exchangers are available for use in adult patients. These tube exchangers are long, semirigid catheters that fit inside ETTs. The Frova Intubating Introducer (Cook Critical Care) is available in a pediatric size (8 F) that allows placement of a 3.0-mm ETT. It is 33 cm in length with a hollow lumen and a blunt curved tip that is shaped like the gum elastic bougie. The blunt curved tip can be passed “blindly” into the trachea when visualization of the glottis is inadequate. The Frova catheter has a hollow lumen and two side ports and is packaged with removable Rapi-Fit adapters that allow ventilation and a stiffening cannula26 (Fig. 36-2).
Cook also manufactures airway exchange catheters (AECs) in four sizes. These catheters are blunt tipped and hollow, with distal side ports and a Rapi-Fit adapter. The 8-F size is 45 cm in length and can be used in 3.0-mm ETTs.26
F Laryngoscopes
2 Oxyscope
The Oxyscope is a fiberoptic Miller no. 1 blade with a port for insufflation of oxygen during intubation. Oxygen insufflation during laryngoscopy in spontaneously breathing, anesthetized infants has been shown to minimize the decrease in transcutaneous oxygen tension, thus making airway instrumentation safer.27
3 Anterior Commissure Laryngoscope
The anterior commissure laryngoscope is frequently used by otolaryngologists for visualization of the glottis. It is a rigid, tubular, straight-blade laryngoscope with a distally located, recessed light source. This design permits enhanced visualization by preventing the tongue from obscuring the field of view.28
4 Bullard Laryngoscope
The Bullard laryngoscope (Circon ACMI, Stanford, Conn), developed by Dr. Roger Bullard at the Medical College of Georgia, is an indirect laryngoscope that utilizes fiberoptic and mirror technology to visualize the larynx.29 Use of fiberoptics and a curved blade enable visualization of the larynx “around the corner” of the blade, thus eliminating the need to align the oral, pharyngeal, and tracheal axes. A standard laryngoscope handle or a flexible fiberoptic cable connected to a light source powers the fiberoptic light source. This laryngoscope is manufactured in three sizes: adult, pediatric, and pediatric long.30 The adult size, with a blade that is 2.5 cm wide, is suitable for children older than 10 years. The pediatric version (newborn to age 2 years) has a blade 1.3 cm wide that extends 0.6 cm beyond the fiberoptics. This blade is recommended for use in neonates, infants, and smaller children. The pediatric long version is available for use in infants and small children up to age 10; it has a longer blade (1.4 cm) and a wider flange (1.6 cm). In the pediatric long version, a multifunctional stylet is attached to the fiberoptic bundle between the eyepiece and handle and aligns the tip of the ETT beneath the flange of the blade. The smallest ETT that passes over the stylet in the pediatric long version is 4.5 mm.30
The Bullard laryngoscope requires minimal mouth opening for its insertion (0.64 cm in cephalad-caudad axis). It has been used to intubate patients with unstable cervical spine or with Pierre Robin, Treacher Collins, Noonan’s, or Klippel-Feil syndrome, among others.29 The adult Bullard laryngoscope has been used successfully to intubate patients older than 12 months with normal airways.31 Contact with the right aryepiglottic fold and, in children, contact with the anterior vocal cord occurred.31,32 Compared with the Wis-Hipple 11/2, the adult Bullard laryngoscope provided a similar view and required a slightly longer time for intubation in children 1 to 5 years of age.32
5 Angulated Video-Intubation Laryngoscope
The angulated video-intubation laryngoscope (AVIL), invented by Dr. Marcus Weiss of Zurich, is an endoscopic intubation device. The AVIL consists of a cast-plastic Macintosh 4 laryngoscope, with the blade angulated distally, and an integrated fiberoptic endoscope (1.8 m long, OD 2.8 mm, VOLPI, Schlieren, Switzerland). The distal blade tip is angulated about 25 degrees to provide increased viewing for the fiberoptic lens. With the angulated tip, the AVIL resembles an activated McCoy blade. Flattening of the blade’s vertical flange enables the device to be used in children. The fiberoptic endoscope runs from the handle to the tip of the blade. The AVIL uses conventional laryngoscopy techniques coupled with video monitoring from the blade tip. Styletted ETTs, in a “hockey stick” configuration, are passed along the vertical flange of the blade under video control.33
The AVIL has been used in patients ranging in age from 3 months to 17 years with manual in-line neck stabilization. In infants and small children, care should be taken with insertion of the blade; initial insertion of the blade was too deep in these patients.34 Several reports document the use of this device in pediatric patients with a DA. The video laryngoscope has been used successfully to intubate children with Morquio’s syndrome as well as a 3-day-old neonate with Pierre Robin syndrome.33,35
6 Truview Laryngoscope
The Truview (Truphatek International, Netanya, Israel) is a recently introduced rigid laryngoscope that has an angulated tip and an optical assembly that provides an illuminated and magnified view of the larynx. The optical system consists of a lens and a prism, which extends the view beyond the tip of the blade. The tip of the device is narrow to accommodate small mouth openings and angulated 46 degrees anteriorly to provide a wider view of the larynx using light refraction. This laryngoscope also has a side port that allows for oxygen insufflation and can be attached to video monitoring to assist with training. The height of the laryngoscope blade is 8 mm. Compared with traditional laryngoscopes, the Truview EVO2 laryngoscope offers various advantages and improves laryngoscopic view. A study by Singh and colleagues35a of 60 neonates and infants comparing the Truview EVO2 with the Miller blade demonstrated an improved laryngoscopic view with the Truview blade, with an increased time to intubation that was statistically but not clinically significant.
7 GlideScope Video Laryngoscope
The GlideScope Video Laryngoscope (Cobalt, Verahon) has a reusable video baton and single-use laryngoscopy blades in two sizes. The laryngoscope comes with a monitor screen, and a video recording unit is also available. The GlideScope Cobalt model features a 10-mm laryngoscope blade. The blade is inserted in the midline without displacing the tongue.36 Two studies have been reported using the GlideScope in children with normal airways. Both studies found it suitable for intubation in pediatric patients.37,38 In one of the studies, the time required for intubation was longer.
8 Airtraq Optical Laryngoscope
The Airtraq Optical Laryngoscope (AOL; Prodol, Vizcaya, Spain) is a single-use indirect laryngoscope for tracheal intubation. The Airtraq comes in two pediatric sizes: infant (size 0) for ETT 2.5 to 3.5 and pediatric (size 1) for ETT sizes 3.5 to 5.5. Both sizes require a mouth opening of 12 to 13 mm. The rubber eyepiece may be used or a camera may be attached and used with a wireless monitor. Images from the distal tip of the blade are projected to the proximal eyepiece. The Airtraq is inserted midline, and the tip may be placed in the vallecula or used to lift the epiglottis. Once the glottis is visualized, the ETT is slowly advanced. For intubation, it is important to lubricate the ETT so that the tube advances easily. Problems with advancement of the ETT may be caused by too large diameter of the ETT, the guide channel, or incorrect angle of the ETT.36 Two case reports documented the use of the Airtraq in pediatric patients with difficult airways: a 9-year-old child with Treacher Collins syndrome who weighed 23 kg37 and a 4.8-kg infant with Pierre Robin syndrome.38 Other case reports have documented difficulty with advancement of the ETT into the trachea despite a good view of the larynx.39
G Stylets
There are various types of stylets available as adjuncts to endotracheal intubation, including the traditional malleable stylet, lighted stylets, and optical stylets. Stylets should be available for the DA. The stylet is inserted into the ETT until the distal end of the stylet is just short of the ETT tip. The ETT and stylet are bent into the desired shape, usually a hockey stick configuration. Complications associated with use of stylets include tracheal trauma, ETT obstruction, and shearing of the stylet. When removal of a stylet becomes difficult, the tip should be examined.40
1 Lighted Stylets
Several different types of lighted stylets, or lightwands, are currently commercially available, including the Vital Signs Light Wand Illuminating Stylet (Vital Signs, Totawa, NJ) and the Tube Stat Lighted Stylet (Xomed, Jacksonville, Fla). Pediatric versions are available for use with ETTs as small as 2.0 to 4.0 mm. The use of the lighted stylet to guide blind endotracheal intubation relies on the principle of transillumination. The presence of a well-defined glow in the neck indicates tracheal placement. Esophageal placement is indicated by the absence of a glow in the neck. Several different reports describe successful intubation of pediatric patients with the lightwand.41,42 Successful technique includes the following principles:
1. A small shoulder roll should be used to keep the head in a neutral to slightly extended position. This is extremely important in a small infant, whose neck naturally flexes when lying on a flat surface because of the large occiput.
2. The lightwand should be advanced in the midline; if the light deviates to one side, the lightwand should be withdrawn and repositioned.
3. The epiglottis is elevated by lifting the jaw with the nondominant hand.
4. Transillumination should be assessed before advancing the lightwand too far.
5. Blind nasal intubation in children is often easier with the rigid stylet left in place.
6. The wand is bent less sharply than for an oral intubation.
2 Optical Stylets
The first optical stylet, described in 1979, was a Hopkins telescope with a fiberoptic external light source (Karl Storz, Tuttlingen, Germany).43 The Seeing Optical Stylet (SOS) system (Clarus Medical, Minneapolis) is a new, reusable, high-resolution fiberoptic endoscope with a malleable stainless steel stylet.36 It combines the features of an FOB and a lightwand. The Shikani Seeing Stylet is portable, lightweight, and available in pediatric and adult versions. The pediatric version is compatible with ETTs 3.0 to 5.0 mm in size. The SOS can be inserted directly into an ETT, allowing intubation to be performed under direct vision. Illumination is provided by a standard green line fiberoptic laryngoscope handle or the included SITElite halogen handle. An adjustable tube stop with an oxygen port, which goes over the shaft of the stylet, allows supplemental oxygen to be delivered. Many factors do not affect the SOS, including cervical spine injury, small mouth, large tongue, and reduced jaw mobility.36
Pfitzner and colleagues44 described the use of the Shikani SOS on eight occasions in seven patients with DA. There were seven successful intubations; one patient, who had previous surgery and radiotherapy for a retropharyngeal rhabdomyosarcoma, could not be intubated by any method. Two patients with limited mouth opening and one patient with a C1-C2 subluxation were intubated on the first attempt. A patient with Hunter’s syndrome was intubated on the second attempt. A potential difficulty mentioned with the SOS is loss of the visual field, which occurs when the lens is next to a mucosal surface. Maneuvers to increase the operating space available are use of a laryngoscope to retract the base of the tongue, lifting the mandible, and pulling the tongue forward.45
The Shikani Stylet is inserted into the ETT after lubrication with silicon spray. The fiberoptic cable can be connected to a video monitor. The mandible is lifted with the left hand and displaced anteriorly until the lower teeth are anterior to the upper teeth.45 The stylet with the loaded ETT is advanced into the trachea under direct vision. Laryngoscopy may be useful in cases of DI (Fig. 36-3). The Shikani Optical Stylet (Clarus Medical) is a portable video stylet.
3 Video-Optical Intubation Stylet
Another video-optical intubating stylet (Acutronic Medical Systems, Hirzel, Switzerland) consists of a flexible fiberoptic endoscope (developed by Dr. Weiss of Zurich). A sliding connector locks the video stylet onto the ETT adapter; it does not require neck extension but does require mouth opening. One report documents successful use of the video-optical intubating stylet in patients age 6 to 16 years, with a simulated grade III laryngoscopic view; 46 of 50 patients were intubated on the first attempt; four attempts were considered failures because of prolonged intubation time (>60 seconds).46
H Laryngeal Mask Airways
1 LMA Family
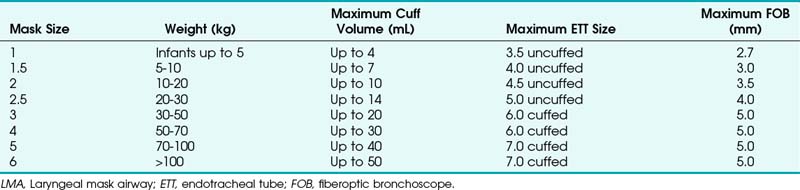
The laryngeal mask airway (LMA North America, San Diego), introduced in 1983 and approved for use in 1991 by the U.S. Food and Drug Administration (FDA), is a standard part of the ASA DA algorithm.1,47 Pediatric versions of the LMA Classic, as well as the disposable LMA, are available for use and are part of the pediatric DA algorithm, as described by Steward and Lerman.48 Application of the LMA requires minimum training and can be useful in neonatal resuscitation.49 The LMA Flexible is available in sizes 2 and 2.5, and the LMA ProSeal is available in a size 2. The size of the LMA in children is determined by the patient’s weight, although a new method has been suggested. With the hand extended and palm side facing up, the thumb and little finger are extended. The second, third, and fourth fingers are placed together. The fully inflated LMA is placed against the palmar side of the patient’s fingers, keeping the widest part of the LMA in line with the widest part of the three fingers. In a study of 163 children at birth to 14 years old, this method was correct in 78%. In the remaining patients, a difference of only one size was observed.50
The LMA has been described as a conduit for blind intubation as well as a conduit for fiberoptic intubation.51–55 Awake placement of the LMA has been described in an infant with Pierre Robin syndrome.56 Anterograde intubation through the LMA with a guidewire was also described in an infant with micrognathia who could not be intubated with conventional methods. A soft-tipped guidewire was advanced through the LMA and the position confirmed by fluoroscopy. An ETT was inserted over the guidewire, followed by removal of the LMA.57 A review of the literature demonstrates different insertion techniques.
The standard technique described with the cuff deflated for adults has also been advocated for children. In addition, a rotational or reverse technique has been described. The LMA is inserted with the cuff facing the hard palate and then rotated and advanced simultaneously. An alternative technique involves inserting the LMA with the cuff partially inflated. Reports on placement of the LMA with the different techniques are conflicting. In children, one study compared two insertion techniques. The partially inflated cuff insertion technique does not increase the incidence of downfolding of the epiglottis and is an acceptable alternative to the standard technique.58 In another study, insertion of the partially inflated LMA required less time and was associated with a higher success rate on first attempts compared with the standard (deflated) technique.59 Results from a study detailing the fiberoptic positioning of the LMA in children with a DA show that 29.5% of patients had a grade I (full) view of the glottis, 29.5% had a grade II (partial) view, and 41% a grade III (epiglottis only) view. Children with a mucopolysaccharide disorder had a grade III view 54% and a grade I view 14% of the time.60
The ProSeal LMA is now available in pediatric sizes. This LMA has a second mask to isolate the upper esophagus with a second dorsal cuff to increase the seal against the glottis. Lopez-Gill and coworkers61 found that it was easily inserted, and oropharyngeal leak pressure was greater than 40 cm H2O (Table 36-3).
I Rigid Ventilating Bronchoscope
The rigid ventilating bronchoscope is extremely useful for ventilating patients with a DA and is included in the most recent version of the ASA DA algorithm as an alternative device in the “cannot ventilate, cannot intubate” situation. In any situation of potential airway collapse, the otolaryngologist and the rigid ventilating bronchoscope should be immediately available (see Chapter 29).
VI Induction Technique
The principles outlined in the ASA guidelines for DA management apply to the pediatric patient. Evaluation, recognition, and preparation are key elements.1 Preoxygenation of pediatric patients, although difficult, should be attempted before any DA intervention, if possible. Studies have demonstrated that the optimal time for preoxygenation in pediatric patients is different from that in adults. Values ranging from 80 to 100 seconds have been reported for adequate preoxygenation in healthy children.62,63 Summoning help early, using awake intubation, and preserving spontaneous ventilation during intubation attempts are also important when managing the DA. The awake or awake sedated approach is preferred in most circumstances when managing the DA. However, in pediatric patients, the patient’s cooperation may limit the usefulness of awake intubation. One well-tolerated technique is placement of a lubricated LMA in awake infants, which provides an airway for inhalational induction.56
The traditional approach to the difficult pediatric airway has been maintenance of spontaneous ventilation under inhalational anesthesia. Premedication with oral or intravenous atropine (0.01-0.02 mg/kg) is indicated for vagolytic and antimuscarinic effects. Inhalation induction may be performed with sevoflurane in 100% oxygen. Sevoflurane has been used in the management of the DA with success.64,65 The low blood gas solubility of sevoflurane and consequent rapid induction and emergence are advantageous when managing the DA. When the ability to ventilate the patient by mask is demonstrated, a small dose of muscle relaxant or propofol may be given to facilitate intubation.
For patients who can tolerate an awake sedated intubation technique, a variety of drugs can be used. One must always keep in mind the risk/benefit ratio when sedating a patient with a DA. Sedatives may further compromise an airway. Sedatives should not be given to any patient in acute distress or with the potential for acute obstruction. Use of sedatives should be based on careful physical examination, anesthesiologist experience with agents involved, and overall patient condition. If no other options are available, slow titration of pharmacologic agents to effect, without loss of spontaneous ventilation, should be performed. Use of pharmacologic agents that are easily antagonized is recommended. For older children and adolescents, a combination of midazolam and fentanyl may be used. Remifentanil can also be used. Dexmedetomidine has been used successfully to perform an awake fiberoptic intubation in a morbidly obese patient with facial, cervical, and upper thoracic edema.66 In extreme circumstances, parental presence at induction may be allowed. Careful preparation of the parent must be performed prior to induction. As soon as the patient separates or begins to lose consciousness, a designated member of the operating room (OR) staff should immediately escort the parent out of the OR.
Another important aspect for successful airway management is topicalization of the airway with local anesthesia. In pediatric patients, this may be obtained by nebulizing, spraying, or swabbing local anesthetic solution or by applying viscous gel to a gloved finger. FOB with suction ports can be used to spray local anesthesia on the vocal cords under direct vision. The maximum dose of local anesthetic allowed should be calculated before topicalization. The drug of choice is lidocaine because it has the best safety profile. Maximum doses of lidocaine are 5 mg/kg. Agents containing benzocaine (e.g., Cetacaine spray; Americaine ointment; Hurricaine ointment, gel, or spray) should be avoided in infants and young children because of the risk of methemoglobinemia.7
VII Airway Management Techniques
A Techniques for Ventilation
Obstruction of the upper airway is a common occurrence in pediatric patients undergoing an inhalation induction. Techniques for overcoming this type of obstruction include insertion of an appropriate-size oropharyngeal airway or a nasopharyngeal airway, or both. Another common mistake is occlusion of the submandibular space with incorrect placement of the anesthesiologist’s hand. Care should be taken to position the hand on the tip of the mandible and not on the submandibular space. Chin lift or jaw thrust combined with continuous positive airway pressure (CPAP) at 10 cm H2O has been shown to improve upper airway patency.67
Additional techniques are available for mask ventilation. The two-person technique involves either one person holding the mask with both hands while an assistant compresses the reservoir bag or a second person assisting in jaw lift while the first person continues to compress the reservoir bag. Another option is using the anesthesia ventilator to provide ventilation so that one person can hold the face mask with both hands.68
B Techniques for Intubation
1 Direct Laryngoscopy
Tips for successful visualization of the larynx include proper use of external laryngeal pressure and positioning. Direct laryngoscopy involves alignment of the oral, pharyngeal, and laryngeal axes in order to visualize the glottis. Because the larynx is situated in a more cephalad position and the occiput is large, the sniffing position in infants does not assist in visualization of the larynx.4,7 The infant should be positioned with the head in a neutral position with the neck neither flexed nor extended.69 A small shoulder or neck roll may be beneficial. Optimum external laryngeal manipulation (OELM) should also be used with a poor laryngoscopic view to improve visualization. OELM may improve the laryngoscopic view by at least one whole grade in adults. This is not cricoid pressure but rather pressing posteriorly and cephalad over the thyroid, hyoid, and cricoid cartilages. Benumof and Cooper70 suggest that OELM should be an instinctive and reflex response to a poor laryngoscopic view. This maneuver has also proved effective in pediatric patients.71 The main mechanism seems to be shortening of the incisor-to-glottis distance.
The two-anesthesiologist technique involves manipulating the larynx under direct vision by the laryngoscopist and intubation by a second anesthesiologist. This technique has been used successfully to intubate a 6-month-old infant with Pierre Robin syndrome and concomitant tongue-tie (ankyloglossia).72
The retromolar or paraglossal technique has been advocated as useful in cases of DI related to a small mandible.73 A straight laryngoscope blade is introduced into the extreme right corner of the mouth overlying the molars, thus reducing the distance to the vocal cords. It is advanced in the space between the tongue and lateral pharyngeal wall until the epiglottis or glottis is visualized. The head is rotated to the left to improve visualization while applying external laryngeal pressure displacing the larynx to the right. Advancement of the ETT is facilitated by retracting the corner of the mouth to allow placement of the ETT. The styletted ETT should be shaped into the classic hockey stick configuration. An alternative approach involves placement of the ETT from the left side of the mouth.74 Lateral placement of the laryngoscope blade reduces the soft tissue compression because the tongue is essentially bypassed. The maxillary structures are also bypassed by the lateral blade placement, thus improving the view.4 Because there is a reduced space for displacement of the tongue in syndromes with micrognathia, this approach may be useful. The retromolar technique has been described as an alternative method for intubation of patients with Pierre Robin syndrome.75 A pediatric version of the Bonfils Retromolar Intubation Fiberscope is the Brambrink Intubation Scope (Karl Storz). It is an optical stylet that allows a retromolar approach to the DA.26
In adults the left molar approach with a Macintosh blade and OELM has been reported to improve the glottic view in cases of difficult laryngoscopy.76 Suspension laryngoscopy is often employed by otolaryngologists as an alternative technique for visualization of the difficult larynx. Intubation of an infant with Goldenhar’s syndrome was accomplished by suspension laryngoscopy.77 This method is similar to standard laryngoscopy by the retromolar technique.
2 Blind Intubation Technique
a Blind Nasotracheal Intubation
Blind nasotracheal intubation requires preservation of spontaneous ventilation either under general anesthesia or with adequate vasoconstriction and topicalization of the nasal mucosa. The tip of the ETT is directed toward the larynx by listening to the intensity of the breath sounds or by the capnograph tracing. This technique requires extensive practice before use. Tips for this technique include external pressure on the neck, which may direct the glottis toward the ETT; placement of a stylet through the ETT after passage through the nasopharynx to direct the tip to the glottis; inflating the ETT cuff with air to center it at the glottis and then deflating it for actual passage; and repositioning of the head (flexion/extension) if the initial intubation attempt fails.48 Pediatric patients with enlarged adenoids may be at risk for bleeding and trauma with this technique. Blind nasotracheal intubation of a neonate with Pierre Robin syndrome has been described.78
b Digital Endotracheal Intubation
Digital intubation is a blind technique that is relatively easy to learn. Intubation of an 8-day-old, 3.3-kg neonate with Pierre Robin syndrome has been reported.79 The left index finger is passed midline along the surface of the tongue until it passes the epiglottis. The left thumb may apply cricoid pressure to steady the larynx. The ETT, using the left index finger as a guide, is advanced into the trachea. This technique has been used as the primary method of intubating neonates in some neonatal centers.80 As with any new technique, practice is required.
c Lightwand Intubation
Intubation with a lightwand is a blind technique that has found success in management of the difficult pediatric airway.41,42 The success rate increases with experience; practice is thus required. As with any new technique, experience with patients who have normal anatomy should be gained first. Contraindications to the use of the lightwand include tumors, infections, trauma, and foreign bodies of the upper airway.37 Causes of failed intubation include entrapment in the vallecula or the aryepiglottic folds. A shoulder roll helps to extend the head and neck and increase the exposure of the anterior neck. After preparation of the lightwand, the jaw is lifted with the nondominant hand or a laryngoscope blade. The lightwand is inserted in the midline into the patient’s mouth, rotated around the patient’s tongue, then gently rocked back and forth. If the ETT wand is in the trachea, a well-defined bright light (size of a quarter) is visible at the level of the subglottis on the anterior surface of the neck.
3 Fiberoptic Laryngoscopy
Aids for fiberoptic intubation (FOI) include face masks, oropharyngeal airways, guidewires, and the LMA.81 The Frei mask previously described or variations of commercially available masks have been used with success.15,16,82 The Patil-Syracuse mask is available in a size 2, but it is difficult to achieve a good seal with this mask. An endoscopy mask can be made by attaching a swivel fiberoptic bronchoscope (FOB) adapter to a pediatric face mask in one of two ways81: a commercially available swivel adapter (Instrumentation Industries, Bethel Park, Pa) can be attached directly to the mask, or an adapter designed for attachment to the ETT (e.g., Portex bronchoscope adapter) can be connected to the face mask with a 15-mm to 22-mm adapter.
Oropharyngeal airways may also be modified for use in pediatric FOI. A strip may be cut from the convex surface of a Guedel-style airway to produce an aid for oral fiberoptic laryngoscopy, creating a channel. The fiberscope is placed in the channel, which helps maintain a midline position. The use of a smaller airway than predicted is suggested so that one may visualize the base of the tongue and epiglottis. Modified oropharyngeal airways are not effective as bite blocks, and one must be careful.81 Also, a nipple from a baby bottle has been modified to act as a conduit for FOI in an infant with an unstable cervical spine. In this case, a hole was cut obliquely into the end of the nipple. After topicalization of the airway with 2% lidocaine, FOI was performed with a 4.0-mm uncuffed ETT.83
Flexible fiberoptic laryngoscopy is one of the cornerstones of DA management. Preparation for fiberoptic laryngoscopy should include preparation of the patient (antisialogue) and checking of the FOB, light source, and suction as well as standard airway equipment. An assistant is necessary for monitoring of the patient and providing a jaw lift, which is useful because it elevates the tongue from the posterior pharynx.7 For older children and adolescents who will be sedated for the procedure, explanation and reassurance in a calm manner are helpful. A method of delivering oxygen is necessary as well. This can be accomplished in a variety of ways, either blowby from the anesthesia circuit or by nasal cannula. For patients who are anesthetized, an LMA or an endoscopy mask may be used to ventilate the patient while the intubation is being performed. Tips for successful oral intubation include midline placement of the FOB, advancement of the FOB only when recognizable structures are visualized, and retraction of the tongue with gauze or clamps if needed.7 If the view from the fiberscope is pink mucosa, the FOB is slowly pulled back until a recognizable structure is seen. If the nasal route is chosen, a topical vasoconstrictor may be used to reduce the chance of bleeding. In a series of 46 patients with DA, fiberoptic nasal intubation was successful on the first attempt in 37 patients (80.4%) and on the second or third attempt in 7 patients (15.2%). Two failures occurred: one related to bleeding and the other to inability to introduce the scope nasally.84
Flexible fiberoptic laryngoscopy may be performed in a variety of ways. The standard technique involves passage of the ETT over the FOB. The ultrathin fiberoptic laryngoscope with a directable tip allows FOI to be performed with ETTs as small as 2.5 mm. Intubation of a 3-month-old infant with the Pierre Robin syndrome has been successfully performed with an ultrathin fiberscope.85 A new 2.5-mm ultrathin flexible FOB with a 1.2-mm suction channel has been used to intubate a newborn with a DA.86 This FOB has a 2.5-mm OD, 1.2-mm working suction channel, angle of deflection of 160 degrees up and 130 degrees down, and a working length of 450 mm.
In scenarios where the available bronchoscope is too large for the required ETT, a staged technique may be employed.87 A FOB with a working channel, a cardiac catheter, and a guidewire are required. The guidewire is passed into the working channel of the fiberscope before intubation. The FOB guidewire assembly is then introduced into the mouth and positioned above the larynx. The guidewire is advanced into the trachea under direct visualization, followed by removal of the FOB. A cardiac catheter (used to stiffen the wire) is threaded over the guidewire. Finally, an ETT is advanced into the trachea over the guidewire-catheter assembly, which is then removed. A modification of this technique involves passage of the ETT over the guidewire without the reinforcing cardiac catheter. This has been used to intubate nasotracheally a 3-day-old infant with Pierre Robin syndrome.88
The fiberoptic bronchoscope may also be used as an aid for nasal intubation either under direct vision or with a guide. In these cases, FOB is introduced into one of the nares while the ETT is advanced into the trachea through the other naris.38 Alternatively, if the ETT cannot be manipulated into the glottis, a guide may be placed in the opposite naris and directed into the trachea. The ETT is then removed and threaded over the guide. A urethral catheter has been used in this manner to assist in the intubation of a 2-week-old neonate with Klippel-Feil syndrome, occipital meningocele, and microretrognathia.89 Another variation of the staged technique involves placement of a larger ETT into the larynx under fiberoptic visualization, followed by removal of the FOB, leaving the larger ETT in the larynx. A bougie is placed through the larger ETT into the trachea, and the ETT is removed. An appropriate-size ETT is then advanced over the bougie into the trachea.90
Flexible FOB intubation through the LMA has been successful.52,53,91 Staged intubation techniques involving the LMA, FOB, guidewires, and catheters (dilators) have been reported, including the use of LMA-assisted wire-guided fiberoptic endotracheal intubation. In a series of 15 cases, Heard and colleagues92 demonstrated that this technique was safe, successful, and easy to learn. After the FOB is placed through the LMA and the vocal cords are visualized, the guidewire is passed through the suction port of the bronchoscope and into the trachea. The LMA and FOB are carefully removed, and the ETT is advanced over the wire. A variation of this theme involves fiberoptic visualization of the glottis through the LMA followed by passage of a guidewire through the suction port of a FOB into the trachea as before. The fiberscope is then removed and an airway catheter or a ureteral dilator passed over the wire into the trachea through the LMA. The LMA is then removed and an ETT advanced over the catheter into the trachea.93 This technique has been used successfully to manage the airway in children with mucopolysaccharidoses. The use of an LMA, an airway exchange catheter (AEC), and a 2.2-mm–OD FOB has also been described.94 After placement of the LMA and visualization of the vocal cords, the fiberscope is removed. The fiberscope is placed into the lumen of a size 11 AEC, which had been cut to 25 cm. This combination was advanced through the LMA into the trachea by a connector. The LMA and FOB are removed, and an ETT is advanced over the Cook AEC.
Przybylo and coauthors95 reported the performance of a retrograde FOI through a tracheocutaneous fistula in a child with Nager’s syndrome. The ultrathin FOB was passed through the fistula in a cephalad direction past the vocal cords and exiting the nares. The ETT was then advanced over the FOB into the trachea.
4 Bullard Laryngoscope
The pediatric Bullard laryngoscope is placed into the oropharynx in the horizontal plane. After passing the tongue, the handle is rotated to a vertical position. One must be careful to stay in the midline as the blade slides around the tongue. The handle then is lifted to visualize the glottis.29 Once the glottis is visualized, a styletted ETT is advanced under direct vision into the trachea.
5 Dental Mirror–Assisted Laryngoscopy
The dental mirror can be used as an adjunct to direct laryngoscopy in order to view an anterior larynx. After direct laryngoscopy is performed, the dental mirror is inserted on the right side of the mouth and angled so that the vocal cords are seen. The handle of the mirror is moved to the left and held by the left hand. An appropriately shaped, styletted ETT then is advanced into the trachea while looking at the dental mirror. Practice is required to develop the necessary coordination. A Stortz no. 3 dental mirror and a Macintosh no. 1 laryngoscope have been used to intubate a 3.9-kg, 2.5-month-old infant.96
6 Retrograde Intubation
The classic retrograde technique involves percutaneous placement of an intravenous catheter through the cricothyroid membrane into the trachea followed by placement of a guidewire. The guidewire exits the mouth or nose and the ETT is then exchanged over the guidewire. If resistance to ETT passage occurs, counterclockwise rotation of the ETT may facilitate placement. This technique has been used for intubation of an infant with Goldenhar’s syndrome.97 A 14-F retrograde intubation set is commercially available from Cook for use with ETTs of ID 5.0 mm or greater.
A combined technique using the FOB and retrograde intubation has been used successfully in management of the difficult pediatric airway as well, as previously mentioned.98
A fiberoptic bronchoscope with a working channel is necessary for the combined technique. The guidewire is threaded into the suction port of an intubating FOB that has a preloaded softened ETT on it. The FOB is passed along the guidewire until it is past the vocal cords. When the scope is past the vocal cords, the wire is withdrawn and the ETT correctly positioned. This technique allows passage without obstruction from the arytenoid cartilage or epiglottis. Oxygen insufflation can be performed through the suction port as well, even with the wire in place. Care must be taken to limit flow to avoid tracheobronchial injury from excessive gas velocity. Audenaert and colleagues99 used this technique in 20 patients with DA age 1 day to 17 years and reported no major complications. Retrograde wire-guided direct laryngoscopy has also been reported for airway management in a 1-month-old infant.100 In that patient, attempts to pass a 2.5-mm ETT over the wire itself were unsuccessful, but endotracheal intubation was achieved over the wire with direct laryngoscopy.
7 Emergency Access
Emergency access is divided into the emergency surgical and the emergency nonsurgical airway.1 Emergency surgical airway access is often difficult and requires the presence of a skilled anesthesiologist. It is the last resort in the “cannot ventilate, cannot intubate” arm of the ASA DA algorithm.101 Three procedures are referred to in this category: emergency tracheostomy, emergency cricothyroidotomy, and percutaneous needle cricothyroidotomy. In children younger than 6 years, emergency tracheostomy is usually the procedure of choice because the cricothyroid membrane is too small for cannulation.99 In older children, percutaneous needle cricothyroidotomy is often preferred over a surgical approach because most anesthesiologists can perform this technique rapidly. Also, there is less risk of injury to surrounding structures.4 Emergency cricothyroidotomy kits are available from Cook with 3.5-, 4-, and 6-mm–ID airway catheters.
The emergency nonsurgical airway access includes use of the LMA, esophageal-tracheal Combitube, and transtracheal jet ventilation (TTJV).1 The Combitube is available in a small-adult size and is contraindicated in patients less than 4 feet tall.7 The LMA is useful in the management of the difficult pediatric airway, as stated previously, as an supraglottic airway device or as a conduit for intubation. However, in the presence of glottic or subglottic obstruction, the LMA is ineffective; TTJV is considered the technique of choice in this situation, as reported in two cases for laser endoscopic surgery.102 Caution with TTJV is urged because serious complications may result from its use.103 TTJV below a glottic or subglottic obstruction may result in barotrauma because the pathway for egress of air and oxygen is limited. Tension pneumothorax has been reported with jet ventilation through an AEC in an adult.104
VIII Complications of Airway Management
Complications that result from intubation in adults can occur in the pediatric population as well. Airway injury accounted for 6% of claims in the ASA closed-claims database.105 Four percent of the airway injury claims involved pediatric patients younger than 16 years. The most frequent sites of injury reported were the larynx (33%), pharynx (19%), and esophagus (18%). Injuries to the esophagus and trachea were more frequently associated with DI. Laryngeal injuries included vocal cord paralysis, granuloma, arytenoid dislocation, and hematoma. Pharyngeal injuries included lacerations, perforation, infection, sore throat, and miscellaneous injuries (foreign body, burn, hematoma, and diminished taste).
An oropharyngeal burn related to the laryngoscope lamp occurred in a term baby weighing 3.6 kg who was easily intubated at birth.106 The laryngoscope was switched on before intubation. Lightbulb laryngoscopes, in contrast to fiberoptic laryngoscopes, can reach temperatures that would result in burns to the oropharynx. Filaments may overlap with use, and it is common for two or more coils to touch.106 The resistance of the lamp decreases and the current increases, thus increasing the temperature. Koh and Coleman106 recommend that all lightbulb laryngoscopes be switched on for less than 1 minute; if left on, the temperature of the bulb should be manually checked before intubation.
Difficult intubation accounted for 62% of all esophageal injuries, with most involving esophageal perforation (90%). Esophageal perforation following DI has been reported in a neonate.107
Laryngotracheal stenosis may be classified as glottic, subglottic, or tracheal. Prolonged intubation seems to be the major etiology. The mechanism responsible seems to be ischemic necrosis caused by pressure from the ETT against the glottic and subglottic mucosa. This results in an inflammatory reaction with a secondary bacterial infection and scar formation. Risk factors include too large an ETT, prolonged intubation, repeated intubation, laryngeal trauma, sepsis, and chronic inflammatory disease.108
The incidence of postintubation croup varies from 0.1% to 1%.20,21 Risk factors include age under 4 years, tight-fitting ETT, repeated intubation attempts, duration of surgery exceeding 1 hour, patient’s position other than supine, and previous history of croup. Reports are conflicting concerning the risk from a concurrent upper respiratory tract infection. Classic treatment consists of humidified air, nebulized racemic epinephrine, and dexamethasone. In pediatric trauma patients, absence of an air leak at extubation was the strongest predictor of postextubation stridor requiring treatment.109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree