Chapter 49 Monitoring the Airway and Pulmonary Function
I Introduction
Although most monitoring devices provide useful information, some are easier to use than others. Some data suggest that the devices that are easiest to use and for which the data are easiest to interpret are the most clinically useful devices.1 In many situations, the information provided by monitors that is thought to be straightforward requires a comprehensive understanding of its physiologic basis. For example, although the pulse oximeter provides a straightforward measurement of oxygen (O2) saturation, which can be easily interpreted in most cases, the information in many clinical situations does not accurately reflect the O2 saturation (in the presence of carbon monoxide) or O2 tension (when the patient is severely acidotic or alkalotic).2,3 Interpretation requires an understanding of the patient’s physiology and the method by which the monitor reports the data. The same caveats are true for almost every monitor that is used clinically to guide diagnosis, management, and response to therapy. The clinician must know what monitors are available and when the information provided by the monitor is clinically useful and must know how to interpret the data and understand the limitations of each device.
This chapter describes techniques for monitoring and evaluating the airway, gas exchange, and pulmonary function. It provides an overview of the monitors used to assess the patient and describes specific monitors that are useful in assessing the appropriateness of mechanical ventilatory support. Because the modes of ventilation have changed considerably and the options for providing mandatory and spontaneously initiated modes of ventilation have become available, monitoring pulmonary function and the patient-ventilator interface has become increasingly important.4 The benefits and limitations of each monitoring technique are discussed.
II Monitoring the Airway
A Non-intubated Patient
Monitoring the airway is a critical component of clinical assessment for any patient requiring sedation, analgesia, or ventilatory support. As a result of underlying anatomic abnormalities, physiologic alterations in the level of consciousness, edema of the airway, or administration of respiratory depressants, a patient can develop upper airway dysfunction with life-threatening consequences. Assessment of the airway should include the clinical evaluation that is routinely performed by the anesthesiologist before initiating anesthesia or by the critical care practitioner before performing endotracheal intubation. In many cases, the assessment must be performed rapidly under challenging circumstances, but if possible, the evaluation should include a brief and focused history and physical examination. The patient should be asked about snoring or episodes of airway obstruction during sleep; previous experiences with endotracheal intubation, including difficult intubation, hoarseness after airway manipulation, and hoarseness with exercise; previous lengthy endotracheal intubation; and a history of tracheostomy or tracheal abnormalities, including stenosis or tracheomalacia. Patients with rheumatoid arthritis should be questioned about upper airway problems, particularly those related to potential arthritic changes in the cricoarytenoid joints. Patients who have had previous neck or mediastinal surgery should be carefully evaluated for evidence of unilateral or bilateral vocal cord dysfunction. For patients unable to provide a history, discussion with family members or the nurse caring for the patient, a review of the medical record, or direct observation of the airway and ventilatory pattern while preparing equipment for intubation or other interventions can provide useful information to guide management decisions. In selected patients, a lateral neck radiograph can provide useful information about the upper airway and presence of masses in the airway or epiglottic edema, although in most cases, upper airway compromise necessitates emergent intubation without the benefit of a radiologic evaluation.5
B During Endotracheal Intubation
Although a variety of masks and other devices are available to facilitate ventilatory support without tracheal instrumentation, the patient requiring airway protection or positive-pressure ventilation usually undergoes endotracheal intubation through transoral or transnasal routes or through a surgical airway (see Chapter 17). When endotracheal intubation is required, confirmation of correct placement is essential. The most reliable method to assess the location of an artificial airway within the trachea is direct visualization of the tube passing through the vocal cords at the time of intubation. Physical examination is also important to ensure that both lungs are being ventilated after placement of the airway. Auscultation over the lung fields (particularly the apices of the lungs) and stomach should routinely be performed to assess ETT placement. When the ETT is within the trachea, equal breath sounds should be heard over both lung fields while listening over the apices. Auscultation over the upper lung fields minimizes the likelihood of hearing sounds transmitted from the stomach. For most adult patients, if the ETT is located within the trachea, no breath sounds should be heard over the stomach. Unfortunately, auscultation can be misleading. Occasionally, particularly in children, breath sounds are transmitted to the stomach even when the ETT is properly positioned. For patients with extensive parenchymal lung disease, effusions, or endobronchial lesions, breath sounds may not be heard equally over both lung fields even when the ETT is properly positioned within the trachea.
Other clinical signs can be useful in determining whether the ETT is within the trachea. They include identifying mist within the lumen of the ETT during exhalation, palpation of the cuff of the ETT in the suprasternal notch, and the normal “feel” of a reservoir bag during manual ventilation. Despite the clinical usefulness of these methods, none is infallible, and false-positive and false-negative evaluations have been reported.6
A more reliable monitor for confirming that the artificial airway is within the trachea is identification of carbon dioxide (CO2) in exhaled gas. If the airway is within the trachea and the patient is ventilating spontaneously or receiving positive-pressure ventilation, CO2 should be eliminated by the lungs. The presence of CO2 or measurement of CO2 concentration can be used to determine the location of the ETT. Several devices are available to monitor CO2 in expired gases. In the OR, CO2 can be measured using an infrared device,7 Raman effect scattering, or mass spectrometry. In the ICU, emergency department (ED), or other settings, including out-of-hospital locations, colorimetric techniques can successfully estimate the CO2 concentration, or infrared devices can accurately measure the CO2 concentration in expired gases.8,9 As a result of the ease of use and widespread availability of these devices, the documentation of CO2 in exhaled gas after placement of an artificial airway (i.e., capnography) has become the standard of care in anesthesia practice and is routinely used during emergency airway management in many hospitals and emergency settings. A detailed description of capnography is provided on page 1011. Unfortunately, even these devices can provide misleading information, and the information they provide is not foolproof.10,11
Capnography is a useful monitor to confirm correct placement of the ETT, but it is not uniformly reliable and can be misleading.12 For example, when the patient has been ventilated by mask before intubation, CO2-containing gas may remain in the stomach. A capnograph may indicate the presence of CO2 in the expired gas, but it does not reflect CO2 from the airway. This problem is even more common when capnography is used to monitor the patient who has recently received bicarbonate-containing solutions or has been drinking CO2-containing beverages before placement of the artificial airway. In these situations, CO2 is eliminated from the stomach during the first few breaths provided through the ETT. The presence of CO2 from exhaled gas therefore should be monitored for at least a few breaths. If CO2 continues to be eliminated through the ETT after four or five breaths, endotracheal placement of the tube can be ensured.11 Another problem with capnography when used to confirm ETT placement is that CO2 elimination occurs only if the patient has sufficient cardiac output to deliver CO2 to the lungs. If the patient has suffered a cardiac arrest and cardiac output is very low or absent, no CO2 is delivered to the lung. The capnogram reveals neither a digital display of CO2 from exhaled gas nor, if the CO2 waveform is being monitored, a capnographic display, even when the ETT is within the trachea.13–16 During cardiopulmonary resuscitation, the presence of CO2 in exhaled gas provides confirmation that the cardiac output has improved and CO2 is being eliminated from the lungs. Sometimes, even when cardiac output is inadequate, chest compressions are effective at eliminating enough CO2 from the lungs to confirm ETT placement.
Another technique to confirm placement of the ETT in the trachea at the time of intubation is use of a self-inflating bulb. This technique was advocated as an easy way to confirm the proper position of the ETT in out-of-hospital intubations. The technique uses a bulb that is applied to the ETT. Self-inflation of the bulb within 4 seconds determines that the ETT is in proper position. Although the technique has some proponents, most studies are unable to confirm that this is a reliable method to verify ETT placement.17
When the position of the ETT within the trachea has been confirmed, it is important to assess the exact location of the tube within the trachea to avoid placement too proximal (increasing the risk of accidental extubation) or too distal (endobronchial). Incorrect positioning of the ETT has been associated with several complications, including pneumothorax and death.18 The location of the ETT should be confirmed at the time of placement and be regularly assessed while the artificial airway remains in place, because the position can change even after the ETT is secured. Flexion of the neck moves the ETT toward the carina, and extension moves the tube up toward the vocal cords. In adult patients, flexion and extension of the head changes the position of the ETT tip by as much as 2 cm.18 As the ETT softens or the patient manipulates the ETT with the tongue, the tube position changes. As a result of changes in ETT position, patients are at risk for self-extubation, even when the tube is secured at the mouth and the extremities are restrained.
Several techniques can be used to assess the correct position of the ETT within the trachea. For example, placement of the ETT to a predetermined distance has been advocated as a way to minimize the likelihood of endobronchial intubation. Owen and Cheney suggested that the tube be placed to a depth of 21 cm in women and 23 cm in men when referenced to the anterior alveolar ridge or the front teeth. In their study using this approach, endobronchial intubation was avoided.19 Subsequent studies have not confirmed that this technique prevents endobronchial intubation in critically ill adults or that it is predictive of the relationship between the position of the ETT at the teeth and the tube’s position relative to the carina.20–22
Fiberoptic bronchoscopy has been used to determine proper positioning of the ETT.23 When a flexible fiberoptic bronchoscope is readily available, it can be used to confirm the location of the tip of the ETT within the trachea.24 Because many ORs and ICUs have “difficult airway carts” readily available, the use of the flexible fiberoptic bronchoscope to assist with intubation and confirm the tubes’s location has become more common. The technique is useful, although it is not without some risk. Insertion of the flexible fiberoptic bronchoscope reduces the effective cross-sectional area of the ETT, potentially compromising ventilation and oxygenation.25 Peak inspiratory pressure increases. Partial obstruction of the ETT results in an increase in airway resistance, which may lead to the development of occult end-expiratory pressure and increase the risk of pneumothorax or cause hemodynamic compromise.26 Despite these limitations, in experienced hands, the assessment can be completed rapidly and without complications. It is a particularly useful way of documenting the location of the ETT within the trachea in the patient for whom the specific location of the tube is critically important, such as one with abnormal tracheal anatomy, the patient at risk for obstruction of the right upper lobe bronchus, or one with specific needs related to the planned surgical procedure.
Capnography can be used to identify endobronchial migration of an ETT.27 With distal migration of the tube, the EtCO2 falls. The change is usually associated with an increase in peak inspiratory pressure. These changes, although not always reliable, can provide early evidence of ETT migration because the CO2 changes precede a change in arterial blood gases (ABGs) or other signs of displacement.
Probably the most commonly used method to assess the location of the ETT within the trachea is the routine post-intubation chest radiograph. The distance of the ETT from the carina can be measured from a portable anteroposterior radiograph obtained at the bedside. Although many clinicians have questioned whether the cost of chest radiography warrants its routine use for documentation of ETT placement, it remains the most useful and reliable method to determine the appropriate depth of the ETT within the trachea.20–22
One special clinical situation warrants additional monitoring of the artificial airway. Some patients require placement of a double-lumen tube to facilitate a unilateral surgical procedure of the lung, to provide differential lung ventilation, or to protect one lung from contamination with blood or infected secretions from the other lung. In these cases, proper placement of the double-lumen tube must be ensured. Physical examination alone and other monitoring techniques are usually insufficient to confirm proper positioning. Fiberoptic evaluation is most often required to confirm the ETT position after initial placement and to reevaluate placement after the patient is repositioned for a surgical procedure or while requiring differential lung ventilation in the ICU.28 Direct visualization of the tip of the double-lumen tube and the relationship between the tracheal and bronchial lumens ensures that the tube is in the proper position and that the two lungs are isolated. Other techniques can be used to diagnose malpositioning of double-lumen tubes, although there are few studies that confirm their value. Capnography, which has been useful in identifying endobronchial migration of a single-lumen ETT,27 may provide information about the location of a double-lumen tube, particularly if only one lung is being ventilated at the time of evaluation. Spirometry, which can be obtained from in-line monitoring devices added to the anesthesia circuit or monitoring techniques provided by critical care ventilators, can also provide early detection of double-lumen tube malpositioning.29 As the ETT migrates, expiratory flow obstruction, as can occur with malpositioning of the tube, can be detected as a change in the shape of the expiratory limb of the flow-volume loop. Inspiratory obstruction is best diagnosed by a change in the pressure-volume loop.
C During Weaning
Careful evaluation and monitoring of the patient’s airway are required before and immediately after tracheal extubation. After the patient is weaned from ventilatory support and is being prepared for extubation, the patient’s ability to protect and maintain the airway after tracheal extubation must be assessed, although it can be difficult to do so with the ETT in place. Various clinical criteria have been used to determine whether the intubated patient can protect the airway. The most common criteria are a normal gag response and a strong cough. If the patient gags when the back of the throat is stimulated and coughs during suctioning, most clinicians feel confident that the patient will be able to prevent aspiration after extubation. These criteria, however, have never been subjected to scientific evaluation. Some patients who have a poor gag or cough with the ETT in place are able to handle secretions and to cough effectively after endotracheal extubation. Others, who seem to have a satisfactory cough or gag before extubation, are still unable to protect the airway when extubated. The problem with airway protection may become clinically apparent only when the patient begins to eat, because pharyngeal function may remain abnormal for several hours to days after endotracheal intubation.30 Nonetheless, these criteria continue to be the most commonly used to determine whether the patient can be extubated safely.
Many patients cannot breathe around the occluded ETT because of the increased resistance with the tube in place. As a result, alternative methods have been suggested. The leak test assesses the airway pressure required for a leak to develop around the cuff when positive-pressure ventilation is applied through the ETT with the cuff deflated.31 Although the specific pressure at which the leak develops has not been well correlated with successful extubation, some clinicians require that a leak occur when the airway pressure is low, usually less than 15 cm H2O, before extubation. Unfortunately, some studies, including a systematic review of the literature that included more than 2300 patients, have been unable to confirm the value of the leak test at all nor a specific leak pressure or volume above which extubation is contraindicated.32 If the airway pressure required to identify a leak during positive-pressure inspiration is high, probably 20 to 25 cm H2O, the patient may have sufficient upper airway edema to warrant leaving the ETT in place until the edema resolves.
D After Tracheal Decannulation
Vocal cord function can be impaired after surgery. Postoperative vocal cord dysfunction can be caused by direct trauma at the time of endotracheal intubation or edema. Recurrent laryngeal nerve dysfunction can also occur, most commonly caused by nerve retraction or transection during surgery or direct trauma from high intratracheal pressure transmitted from the ETT cuff.33 Unfortunately, it is difficult to assess vocal cord function with the ETT in place. The evaluation usually requires that the ETT be removed (see Chapter 50). After extubation, evaluation of laryngeal and vocal cord function can be assessed fiberoptically. In some patients, evaluation of the airway can be performed by inserting the fiberoptic device through the ETT and then removing the tube over the fiberoptic shaft to allow visualization of the airway. Assessment requires that the patient breathe spontaneously, so that the movement of the vocal cords can be visualized. Although the assessment can be performed in the ICU, the more common approach is to perform the evaluation under more controlled conditions in the OR, where all of the emergency airway and surgical equipment is available to secure the airway. Evaluation and extubation can be performed after the patient is anesthetized with a volatile anesthetic agent and is breathing spontaneously. If severe stridor or airway obstruction develops with removal of the ETT, the patient can be reintubated or have a tracheostomy performed for long-term airway maintenance (see also Chapter 31). In most cases, even when there is injury to a recurrent laryngeal nerve or one vocal cord, the patient is able to breathe normally without stridor, unless the patient’s inspiratory flows are excessive. The greater risk exists for the patient who suffers bilateral vocal cord palsies. While still sedated, the patient may not have stridor or evidence of airway obstruction. However, as the patient awakens and inspiratory flows increase, the stridor becomes obvious and usually requires emergent endotracheal intubation or, more commonly, tracheostomy.
III Monitoring Respiratory Function
A Clinical Assessment
The clinical examination remains one of the most important and valuable methods to monitor a patient’s respiratory status. Too often, attention is placed on technologically sophisticated monitoring devices, and the physical examination is cursory, or the clinical findings are undervalued. Nonetheless, much information about actual or potential airway problems and abnormalities in pulmonary mechanical function or gas exchange can be obtained from a carefully performed and thorough examination. Many of the early signs of respiratory failure are apparent on physical assessment (see Chapter 9) before the abnormalities are apparent by other means. For example, the respiratory rate provides important information about respiratory reserve, dead space, and respiratory drive, particularly when interpreted in conjunction with arterial carbon dioxide tension (PaCO2). Tachypnea is frequently the earliest sign of impending respiratory failure. The patient’s pattern of breathing should be evaluated. Subtle changes in the respiratory rate, VT, and pattern of breathing may provide an early indication of increased work of breathing (as may occur with reduced lung compliance, increased airway resistance, or phrenic nerve dysfunction) or altered ventilatory drive. Although inspiratory flow and minute ventilation (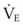 ) are difficult to quantify by clinical examination alone, respiratory distress often manifests as the patient attempts to increase alveolar ventilation by taking larger, more rapid inspirations.
) are difficult to quantify by clinical examination alone, respiratory distress often manifests as the patient attempts to increase alveolar ventilation by taking larger, more rapid inspirations.
Upper airway obstruction, as may occur after manipulation of the airway, in association with epiglottitis or a mass in or around the airway can be assessed by careful clinical evaluation. Nasal flaring, stridor, and chest wall movement in the absence of airflow suggest upper airway obstruction. If the patient is making respiratory efforts and has abdominal expansion during inspiration without chest excursions, he or she has upper airway obstruction and may require manipulation of the upper airway, including a jaw thrust, initiation of positive-pressure ventilation support with continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BiPAP), and endotracheal intubation. When the patient presents with stridor, the physical evaluation is also useful in identifying the location of airway compromise. When the stridor occurs primarily during inspiration, it is caused by extrathoracic obstruction; when it occurs during exhalation, it reflects an intrathoracic obstruction. If the stridor occurs during both inspiration and exhalation, the obstruction is fixed, such as may occur with tracheal stenosis. The fixed obstruction is rarely amenable to conservative treatment, and endotracheal intubation is most likely to be required until a more definitive therapy can be provided. In selected patients, helium therapy can be used as a temporizing intervention until a more definitive treatment can be provided.34
Respiratory dyssynchrony is an early and critical indicator of respiratory muscle fatigue and impending respiratory failure.35,36 Respiratory dyssynchrony (when the patient has no evidence of upper airway obstruction) is identified by assessing chest wall and abdominal movement during normal tidal breathing. A paradoxical respiratory pattern suggests that the patient may have inadequate muscle strength to sustain spontaneous respiration and that positive-pressure ventilation support may be required. Tobin and colleagues found that respiratory muscle dyssynchrony could occur before the development of fatigue,37,38 although fatigue of the respiratory muscles did not always result in the development of dyssynchrony.39
Clinical observation of the patient should include careful assessment of the respiratory muscles as a way of assessing the patient’s respiratory reserve. Use of accessory muscles, including the sternocleidomastoid and scalene muscles, is commonly seen in patients with long-standing respiratory failure associated with chronic obstructive pulmonary disease (COPD).40 The position of the diaphragm and diaphragmatic motion are also affected in patients with severe COPD. The patient who relies on accessory muscles and has minimal diaphragmatic excursion does not have any respiratory reserve. The patient is at risk for recurrent respiratory failure and presents a significant challenge during weaning when mechanical ventilatory support is required.
B Radiologic Evaluation
The chest radiograph is another important monitor of the pulmonary status, although it represents a static picture of the clinical situation. The chest radiograph can confirm proper placement of central venous and other catheters, the ETT,22 and pacemakers. Routine portable chest radiography usually provides evidence of pulmonary infiltrates and pulmonary edema. Radiographic findings that suggest pulmonary edema include bronchial cuffing, perihilar pulmonary infiltrates, and Kerley B lines. Although these findings are helpful, in many critically ill patients, differentiation of diffuse, bilateral infiltrates caused by infection from pulmonary edema can be difficult. When underlying pulmonary diseases such as COPD coexist with acute pulmonary edema, the classic bilateral, fluffy pulmonary infiltrates may not be present. In these circumstances, the x-ray findings must be correlated with other clinical data to explain the radiographic findings.
C Assessment of Gas Exchange
One of the most important goals in monitoring pulmonary function is to determine whether the lung is able to sustain satisfactory oxygenation and ventilation. Invasive and noninvasive monitors of gas exchange are used routinely. Although noninvasive devices are useful and provide important information about oxygenation and ventilation, the ABG determination remains the most frequently used monitor of oxygenation, ventilation, and acid-base abnormalities.41
1 Blood Gas Monitoring
Although monitoring gas exchange using ABG measurements is important, the technique has some limitations. Blood gas monitoring is invasive, and samples must be drawn from an indwelling arterial catheter or an arterial puncture. Frequent blood gas sampling can result in significant blood loss, which may be a clinical problem for any unstable patient, particularly the pediatric patient or anemic adult. Placement and maintenance of an arterial catheter have associated risks, including hemorrhage, hand ischemia, arterial thrombosis and embolism, infection,42,43 and development of a radial artery aneurysm.44
Blood gas monitoring usually is obtained by intermittent sampling from an arterial puncture or indwelling arterial catheter. When a patient’s respiratory status is unstable or is rapidly evolving or when frequent adjustments in ventilatory support are required, intermittent monitoring may be insufficient. In these clinical situations, continuous monitoring is preferable. Continuous intra-atrial blood gas monitors can provide useful real-time data regarding gas exchange and acid-base status,45,46 although the clinical utility of these monitors has not been validated, and the technology is not widely available.47,48 These monitors use fluorescence-based probes placed through an arterial catheter to provide a continuous assessment of PaO2, PaCO2, and pH. The information obtained from these instruments should provide more immediate information about changes in gas exchange or acid-base balance. However, the probes and monitors are more expensive than intermittent blood gas analysis and have not become routine monitors.
2 Noninvasive Monitoring
Assessment of gas exchange using noninvasive techniques has revolutionized clinical care, particularly for anesthesiologists and intensive care providers. Because clinical evaluation of gas exchange is unreliable and often a late sign of deterioration,49 noninvasive devices that continuously monitor oxygenation and ventilation are valuable tools. Several noninvasive methods are available for evaluating oxygenation and ventilation. The most commonly used devices include the pulse oximeter for monitoring oxygenation and the capnograph for evaluating ventilation.
a Pulse Oximetry
Pulse oximetry provides a rapid, continuous, and noninvasive estimation of the O2 saturation of hemoglobin in arterial blood, and it is used routinely to monitor clinical care involving airway management in the OR, ED, and ICU.50–53 It has become the standard monitor of oxygenation during administration of sedation for procedures and during general medical care.54–57 With routine use of this monitor, a high prevalence of clinically undetected hypoxemia in adults and children has been demonstrated.50,57–59 These episodes of desaturation may affect morbidity and mortality.60,61 With severe and sustained hypoxemia (i.e., oxygen saturation from pulse oximetry [SpO2] is less than 85% for more than 5 minutes), patients with known cardiac disease were twice as likely to have perioperative ischemia after noncardiac surgery.61 Among medical patients, those who experienced episodes of hypoxemia within the first 24 hours of hospitalization were three times more likely to die 4 to 7 months after discharge.60
It is logical to assume that the routine use of pulse oximetry has made caring for patients safer by increasing the detection of hypoxemia, better understanding its causes, and allowing more rapid and effective interventions to correct the pathophysiologic causes. Some clinicians have suggested that the early detection of arterial oxygen desaturation with the use of pulse oximetry may improve outcomes.61–64 Although clinical studies do not confirm this belief, they do not negate the presumed benefit of this monitoring tool.65–68 A systematic review of the Cochrane database found no evidence of an outcome benefit of the use of pulse oximetry in anesthesia practice.69 Despite the lack of good outcome data to document that value of pulse oximetry, its use is considered standard of care for critically ill patients and patients receiving moderate or deep sedation or anesthesia.
To measure the O2 saturation of hemoglobin in arterial blood, pulse oximetry uses two fundamental principles: the differential light absorption of oxyhemoglobin (O2Hb) and reduced hemoglobin (Hb) and the increase in light absorption produced by pulsatile blood flow compared with that of a background of connective tissue, skin, bone, and venous blood.56,70 The spectrophotometric principle that forms the basis for oximetry is the Lambert-Beer law (Equation 1), which allows determination of the concentration of an unknown solute in a solvent by light absorption.
I1 = intensity of the light out of the sample
I0 = intensity of the incident light
a = absorption coefficient of the substance
l = distance the light travels through the material (i.e., path length)
c = concentration of the absorbing species
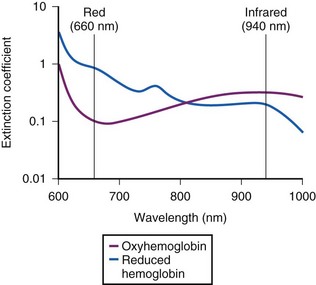
The commercially available pulse oximeters use light-emitting diodes (LEDs) that transmit light at specific wavelengths: 660 nm (red) and 940 nm (infrared). These wavelengths were selected because the absorption characteristics of O2Hb and reduced Hb are sufficiently different at these wavelengths to allow differentiation of O2Hb and Hb (Fig. 49-1).
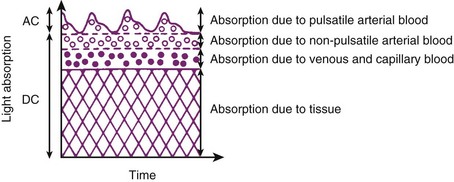
The pulse oximeter determines arterial saturation by timing the measurement to pulsations in the arterial system. During pulsatile flow, the vascular bed expands and contracts, creating a change in the light path length.51 These pulsations alter the quantity of light transmitted to the sensor and provide a plethysmographic waveform.71 This timing of the signal allows the pulse oximeter to differentiate arterial oxygen saturation from venous saturation on the basis of the ratio of pulsatile and baseline absorption of red and infrared light (Fig. 49-2).
The O2 saturation displayed by the pulse oximeter is empirically related to this calculated value on the basis of calibration curves derived for healthy, nonsmoking adult men breathing O2 at various concentrations. Most commercially available pulse oximeters are calibrated over the range of 70% to 100%. The accuracy of pulse oximetry in determining the SaO2 of Hb has been excellent over this range,51 with an error of ±3% to 4%.57
Although pulse oximetry has become a ubiquitous monitoring device, particularly to confirm adequacy of oxygenation during airway management, it has limitations. First, the measurement of O2 saturation does not provide a direct assessment of oxygen tension. Because of the shape of the oxygen-hemoglobin dissociation curve, at higher levels of oxygenation measurements of SpO2 are insensitive in detecting significant changes in PaO2 (Fig. 49-3). Second, the pulse oximeter is not accurate when oxygen saturation is less than 70%. The inaccuracy results from the limited range of O2 saturations used in the calibration process and the difficulty in obtaining reliable human data at these low oxygen saturations.71,72
The accuracy of pulse oximeters during hypoxemia has been extensively studied and reviewed.37,73–76 Most of these studies have been performed on healthy volunteers who had desaturation induced by breathing hypoxic gas mixtures for short periods. Pulse oximeters from different manufacturers varied in their accuracy during hypoxemia; the direction of error differs among these devices, with some overestimating and some underestimating true arterial O2 saturation. Some study results documented problems with the calibration curves and caused revision of the algorithms by the manufacturers.72–77 These modifications to the algorithms have improved performance of the oximeters.57
Other factors affect the performance of pulse oximeters. The response characteristics of pulse oximeters are clinically important, particularly in situations in which the saturation may be changing rapidly, as can occur during management of the difficult airway. Investigators have studied the response characteristics of pulse oximetry in clinical practice.72–77 West and colleagues studied five obese, nonsmoking men with sleep apnea syndrome.78 During spontaneous desaturation, the pulse oximeter underestimated the minimum SaO2, and during spontaneous resaturation, there was an overshoot of the maximum SpO2. The location of the probe also influences the response time for the pulse oximeter. Probes placed on the ear respond more quickly to sudden decrease in SaO2 than probes placed on a digit.75 The response time to changes in O2 saturation of the pulse oximeter also depends on heart rate. For fingertip sensors, as heart rate increases, the response to an acute change in saturation is faster; for ear or nasal probes, the relationship is reversed, and as heart rate increases, the response to changes in SaO2 is slower.78
Accuracy of the pulse oximeter is altered in several situations (Box 49-1). Excessive light, such as fluorescent or xenon arc surgical lights, bilirubin lights, and heating lamps, can cause falsely low or high SpO2 values.57,72,79 Covering the probe with an opaque material helps to eliminate this problem. Electrocautery devices can produce significant electrical interference, which results in improper functioning of the pulse oximeter.56 The infrared pulse waves used by neurosurgical image guidance systems interfere with the signal quality and O2 saturation detection by pulse oximetry.80 The use of aluminum foil as a shield was effective in restoring the accuracy of six brands of pulse oximeters when exposed to the infrared signal generated by a neuronavigation device.81 Misalignment of disposable pulse oximeter probes may cause falsely low O2 saturation to be displayed by pulse oximeters, even though the plethysmographic tracing is of excellent quality, and this can change anesthetic management.82 In 100 patients entering the postanesthesia care unit (PACU) at Massachusetts General Hospital, only 6 had perfect placement of the probes, and for the remaining 94, the average misalignment distance was 5.4 mm (range, 0 to 23 mm).82 In a single case report, a Massimo Signal Extraction Technology (Massimo SET; Irvine, CA) pulse oximeter using SatShare technology with a Datex-Ohmeda AS/3 monitor displayed an uninterrupted waveform and normal O2 saturation during asystole in a patient undergoing abdominal surgery.83
Box 49-1 Conditions Affecting the Accuracy of Pulse Oximetry
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



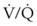 ) scans have been used to detect pulmonary emboli, although the
) scans have been used to detect pulmonary emboli, although the  scans are often inadequate or impossible when assessing a mechanically ventilated, critically ill patient for possible pulmonary emboli. For the ICU patient with suspected pulmonary emboli, pulmonary arteriograms or, more commonly, spiral CT scans are performed because they can be completed quickly and provide better diagnostic information than the
scans are often inadequate or impossible when assessing a mechanically ventilated, critically ill patient for possible pulmonary emboli. For the ICU patient with suspected pulmonary emboli, pulmonary arteriograms or, more commonly, spiral CT scans are performed because they can be completed quickly and provide better diagnostic information than the 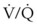 scan alone.
scan alone.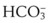 ), oxygen saturation (Sa
), oxygen saturation (Sa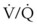 mismatch, shunt, or inadequate cardiac output (i.e., low mixed venous oxygen tension [
mismatch, shunt, or inadequate cardiac output (i.e., low mixed venous oxygen tension [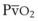 ]). Documentation of an acceptable Pa
]). Documentation of an acceptable Pa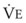 is appropriate and is not an indication of respiratory failure. Similarly, the patient who has a significant metabolic acidosis should increase
is appropriate and is not an indication of respiratory failure. Similarly, the patient who has a significant metabolic acidosis should increase 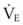 to normalize the pH. In interpreting whether the patient is ventilating appropriately and has a normal ventilatory drive, the Pa
to normalize the pH. In interpreting whether the patient is ventilating appropriately and has a normal ventilatory drive, the Pa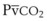 is small, and
is small, and 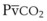 can be used as an estimate of Pa
can be used as an estimate of Pa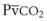 cannot be used as a substitute for Pa
cannot be used as a substitute for Pa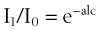 (1)
(1)
 (2)
(2)