
SURGICAL INFECTIONS OF SKIN AND SOFT TISSUE
Infections of skin and soft tissue (SSTI) encompass a diverse set of conditions and are a common cause of hospitalization and morbidity. For the purposes of clinical trials, the U.S. Food and Drug Administration (FDA) uses the term skin and skin structure infections (SSSI), further divided into “uncomplicated” and “complicated” subgroups.1 Necrotizing tissue soft tissue infections (NSTIs) are studied separately and are therefore excluded from studies of complicated SSTI (cSSTI), although certainly not from the practices of acute care surgeons. The term “skin and soft tissue infections” is preferred and used herein as inclusive of the infections that may necessitate surgeon involvement.
New terminology has been proposed by the FDA for the study of antimicrobial therapy of SSTIs: “acute bacterial skin and skin structure infections (ABSSSI).”2 This terminology, not in clinical use, describes infections of lesser severity that do not require surgical drainage or debridement, owing to the perceived difficulty of parsing whether it is the surgery or the antibiotic that is most important to achieve cure. For the purposes of this discussion of surgical therapy of SSSIs, the definition is irrelevant, and the question of efficacy is moot.
SSTI vary widely in severity; whereas some SSTIs can be treated non-operatively, others are life threatening and require radical surgical debridement in addition to broad-spectrum antibiotic therapy. Practice guidelines for treatment of SSTIs have been published by both the Infectious Diseases Society of America (Table 43.1)1 and the Surgical Infection Society (Table 43.2).3
Table 43.1
SUMMARY OF RECOMMENDATIONS FROM PRACTICE GUIDELINE FOR DIAGNOSIS AND TREATMENT OF SKIN AND SOFT TISSUE INFECTIONS (SSTI)-INFECTIOUS DISEASES SOCIETY OF AMERICA
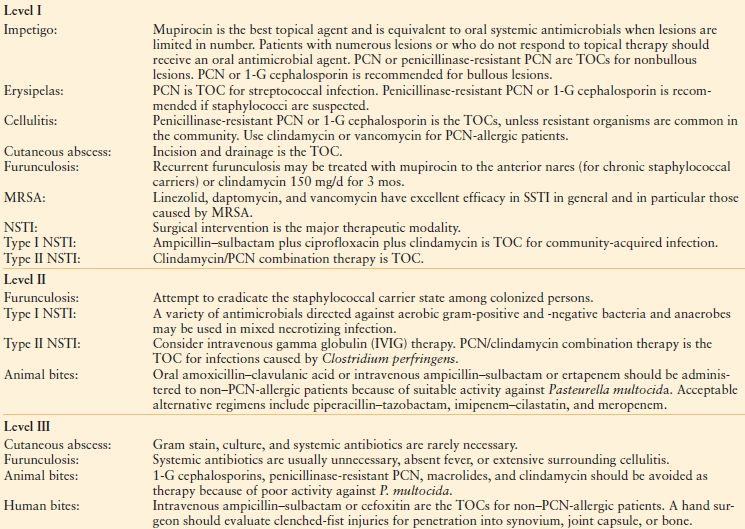
Suspicion of possible surgical site infection does not justify use of antibiotics without a definitive diagnosis and the initiation of other therapies, such as opening the incision. All infected surgical incisions should be opened.
1-G, first-generation cephalosporin; MRSA, methicillin-resistant Staphylococcus aureus; NSTI, necrotizing soft tissue infection; PCN, penicillin; SSI, surgical site infection; TOC, treatment of choice.
From Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41: 1373–1406
TABLE 43.2
SUMMARY OF RECOMMENDATIONS FROM GUIDELINES FOR TREATMENT OF COMPLICATED SKIN AND SOFT TISSUE INFECTIONS FROM THE SURGICAL INFECTION SOCIETY

aNote that telavancin is FDA-approved for the treatment of SSTI caused by MRSA, but was approved subsequent to publication of this guideline
CA-MRSA, community acquired methicillin-resistant Staphylococcus aureus; CT, computed tomography, FDA, U.S. Food and Drug Administration; HBO, hyperbaric oxygen therapy; MRI, magnetic resonance imaging; MRSA, methicillin-resistant Staphylococcus aureus; NSTI, necrotizing soft tissue infection; SSTI, skin and soft tissue infections, TOC, treatment of choice.
From May AK, Stafford RE, Bulger EM, et al. Treatment of complicated skin and soft tissue infections. Surg Infect (Larchmt). 2009;10: 467–499.
Uncomplicated SSTIs are superficial or self-limited. They may require only incision and drainage (without antibiotics) or oral antibiotics (without drainage) and rarely require hospitalization. Uncomplicated infections have historically been excluded from trials of antibiotic therapy for complicated SSTIs and vice versa, but may be included henceforth under the ABSSSI paradigm.
By contrast, cSSTIs involve deeper tissues or require major surgical intervention. An infection is also considered complicated if the patient has specific medical comorbidities, including chronic kidney disease, diabetes mellitus, or peripheral arterial disease. Examples of cSSTI include major abscesses, deep (organ/space) surgical site infections (SSIs), diabetic foot infections (DFIs), incisional SSIs with systemic signs of infection, infected decubitus ulcers, and NSTIs. Because clinical trials of drug therapy for cSSTI exclude NSTI, limited prospective data have been collected for their treatment.
UNCOMPLICATED SSTI
Uncomplicated infections include simple abscess, impetigo, furuncles, and nonnecrotizing cellulitis. Nonnecrotizing cellulitis is a diffuse, spreading skin infection without a suppurative focus, usually presenting as a complication from a breach of skin integrity.4 The differential diagnosis of cellulitis includes both infectious and noninfectious entities, and serious underlying sources of infection should be ruled out. Cellulitis may be a sign of a deeper infection (e.g., spread of subjacent osteomyelitis, thigh cellulitis following colon perforation into the retroperitoneum). Clinicians must be aware that early NSTIs can be confused for cellulitis if initial symptoms are modest.
Cellulitis is caused most commonly by gram-positive cocci, either streptococci (usually Streptococcus pyogenes) or Staphylococcus aureus.5 In cases where patients are immunocompromised, causative agents can also include Haemophilus influenzae and S. pneumoniae.3 Predisposing factors include alterations of skin integrity or venous or lymphatic drainage or alterations in host defenses, such as diabetes mellitus. The diagnosis of cellulitis is made clinically, based on history and the appearance of the lesion; neither imaging studies nor cultures of the lesion have a high diagnostic yield. Needle aspiration or punch biopsy also has low yield; an organism is isolated 5%–40% of the time.6–8
Simple cutaneous abscesses are well-circumscribed collections of purulent material within the dermis and subcutis. These lesions are usually painful, tender, and fluctuant, with a central pustule and surrounding erythema and edema. These infections are typically polymicrobial, with S. aureus isolated in only one-quarter of cases. Treatment of cutaneous abscesses is incision and drainage, with mechanical destruction of intracavitary loculations (Table 43.1).1 Gram stain, culture, or systemic antibiotics are rarely necessary unless a patient has systemic signs or severe immune compromise. Antibiotic coverage is usually unnecessary unless the patient has surrounding cellulitis.
In cases where antibiotic treatment is warranted for uncomplicated SSTI, oral therapy is appropriate unless the patient has systemic signs (e.g., fever, chills), medical comorbidity, or a rapidly spreading lesion. Appropriate oral agents include dicloxacillin, cephalexin, cephradine, or cefadroxil. For moderate-to-severe infections, parenteral penicillins are the treatment of choice; however, treatment failures may occur in severe cases. Other possible regimens for initial empiric parenteral therapy of severe nonnecrotizing cellulitis are antistaphylococcal penicillins such as nafcillin or cephalosporins such as cefazolin or ceftriaxone (Table 43.1). If methicillin-resistant S. aureus (MRSA) is suspected or the patient is highly allergic to penicillin (i.e., anaphylactoid reaction), vancomycin, telavancin, tigecycline, or linezolid may be chosen. A new “fifth-generation” cephalosporin with activity against MRSA, ceftaroline (see below), has also been approved, specifically for ABSSSI. Minocycline or linezolid may be appropriate for oral therapy of hospital-acquired MRSA.
MRSA IN SSTI
MRSA in SSTI requires particular mention. Approximately 60% of hospital isolates of S. aureus are MRSA9; hospitalized or recently hospitalized patients who develop a SSTI must be considered for empiric therapy against hospital-acquired strains of MRSA. Community-acquired MRSA (CA-MRSA) SSTIs are being observed increasingly among patients with no prior contact with the health care system.10 Molecular epidemiologic studies indicate that CA-MRSA is a genetically unique pathogen that has a distinct susceptibility pattern. The CA-MRSA clone likely arose from antibiotic selection pressure upon a commensal, saprophytic Staphylococcus sp. that is part of normal skin flora.11 About 75% of CA-MRSA infections are SSTI, but this pathogen may cause pneumonia as well; either manifestation may be associated with tissue necrosis.12 In many areas of the United States, CA-MRSA is now the predominant cause of SSTIs that present to emergency departments and should be considered when choosing empiric therapy.13
The antimicrobial susceptibilities of CA-MRSA differ from the hospital clones. CA-MRSA is similarly susceptible to vancomycin and linezolid, but CA-MRSA may also be susceptible in vitro to macrolides, clindamycin, and co-trimoxazole. Whereas the latter drug is most reliable for oral therapy,14 severe infections usually require intravenous antibiotics.15,16 Macrolide-inducible clindamycin resistance has been associated with treatment failures; therefore, caution is advised with clindamycin therapy,17 especially in the presence of macrolide resistance.
Outbreaks of CA-MRSA have been associated with groups of people in close contact, including prison inmates, amateur and professional sports teams, military recruits, and clients of day-care centers.18–21 Direct contact with skin is a definite risk factor, as are shared personal hygiene items (e.g., towels, bars of soap). Also at risk are young children and people of lower socioeconomic status. SSTI infections caused by CA-MRSA have a characteristic appearance that may aid in diagnosis. The lesions are usually superficial and well-demarcated, often with central necrosis. If uncomplicated, incision and drainage alone or topical mupirocin ointment or chlorhexidine solution may be sufficient therapy. If antibiotic therapy is required, oral co-trimoxazole should be considered, but severe infections are at risk of treatment failure with this regimen and should be treated with a parenteral glycopeptide or linezolid.
Complicated SSTIs involve deeper tissues or require major surgical intervention. Infection in the presence of chronic kidney disease, diabetes mellitus, or peripheral arterial disease also defines a cSSTI. Examples of cSSTIs include major abscesses, organ/space SSIs, some incisional SSIs (those with systemic signs of infection), DFIs, infected decubitus ulcers, and NSTIs. Clinical trials of drug therapy for cSSTI exclude NSTIs because of high mortality rates and the crucial contribution of timely surgical debridement to outcome. An evidence-based summary of recent clinical trials of antibiotic therapy is shown in Table 43.3.1,15,22–34 Most trials are designed to demonstrate “non-inferiority” of the tested regimen against the comparator regimen, and so numerous regimens appear comparable despite widely divergent spectra of activity. Notably, comparable outcomes are achieved by agents that treat only gram-positive cocci (e.g., vancomycin, linezolid, daptomycin, telavancin), but the many options available to investigators (e.g., choice of comparator in “standard therapy” regimens, addition of aztreonam) make the literature a challenge to interpret.
TABLE 43.3
RECENT PROSPECTIVE TRIALS OF ANTIBIOTIC THERAPY FOR COMPLICATED SKIN AND SKIN STRUCTURE INFECTIONS
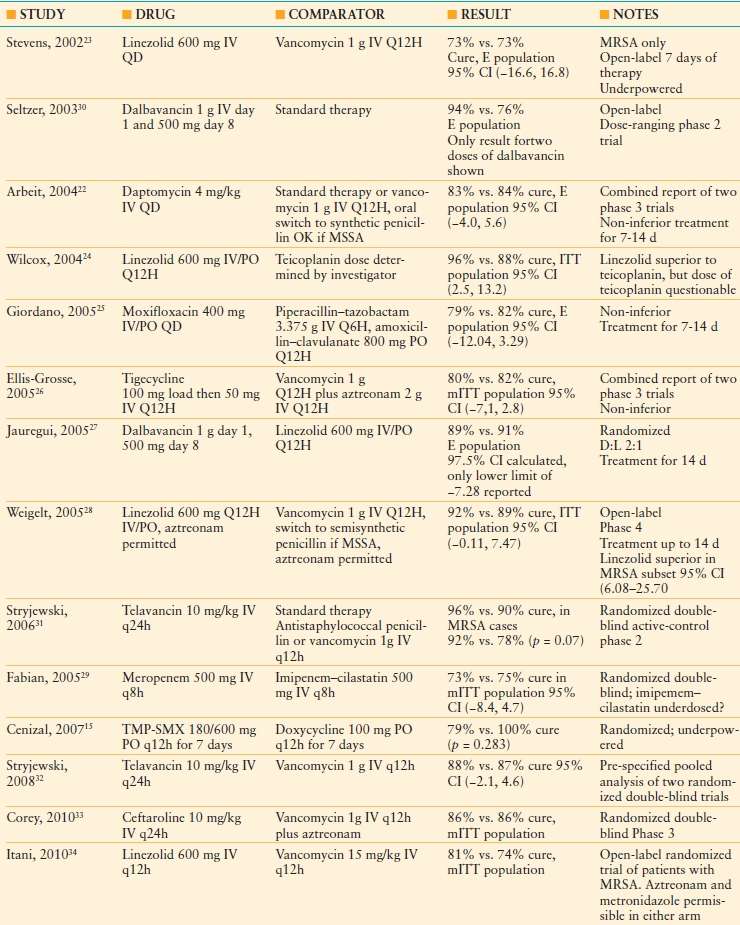
“Standard therapy” means that selection of the comparator agent was at the discretion of the investigator. Non-inferiority is defined statistically when the lower limit of the 95% confidence interval is >-15, and the confidence interval contains zero. A trial with a relatively narrow confidence interval is likely to have greater statistical power (or more homogeneous results) than one with a wider interval.
CI, confidence interval; E, evaluable patient group; ITT, Intention-to-treat patient group; IV, intravenous; mITT, modified intention-to-treat patient group; PO, oral administration; TMP-SMX, trimethoprim-sulfamethoxazole.
Three meta-analyses have been published recently to assess the literature comparing linezolid with vancomycin for cSSTI (n = 2)35,36 and daptomycin versus comparator agents (either vancomycin or semisynthetic penicillins37; Table 43.4). Despite considering different trials for inclusion, the two meta-analyses of linezolid versus vancomycin each concluded that resolution of cSSTI is more likely after linezolid therapy, albeit with more nausea, diarrhea, and myelosuppression (nephrotoxicity is more common with vancomycin). Differences in cure rates of infection were not observed for daptomycin versus comparators.
TABLE 43.4
RESULTS OF META-ANALYSES OF LINEZOLD AND DAPTOMYCIN THERAPY FOR CSSTI
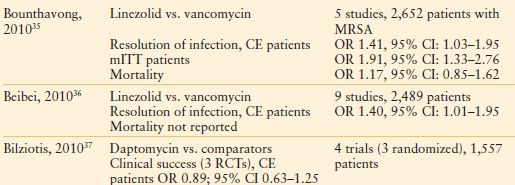
CE, clinically evaluable; CI, confidence interval; cSSTI, complicated skin and soft tissue infections; mITT, modified intent-to-treat population; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratios, RCT, randomized controlled trial.
Diabetic Foot Infection
Patients with diabetes mellitus are at risk for considerable morbidity as a result of chronic foot ulceration and foot infection, including limb loss.38,39 DIFs are usually a consequence of skin ulceration, either from ischemia or trauma to an insensate, neuropathic foot. One-third of patients presenting with a DFi have had a chronic foot lesion for more than 1 month beforehand. Two-thirds of patients with DFi present with peripheral arterial disease, and the prevalence of sensory neuropathy is about 80%. Although most DFIs remain superficial, as many as 25% spread contiguously to involve subcutaneous tissue or bone (osteomyelitis). The compartmentalized anatomy of the foot, with its various spaces, tendon sheaths, and neurovascular bundles, allows ischemic necrosis to affect tissues within a compartment or spread along anatomic planes. Recurrent infections are common, and 10%-30% of affected patients eventually require amputation.40
Diabetic patients are predisposed to DFIs not only because of the portal of entry and poor perfusion, but also because of defective innate immunity, including impaired neutrophil and monocyte/macrophage function, which appear to correlate with the adequacy of glycemic control.41,42 Adaptive immunity may be impaired as well. Diabetic patients also have a higher prevalence of nasal carriage of S. aureus, which predisposes patients to SSTIs.
Infections affect the forefoot most commonly, especially the toes and the metatarsal heads on the plantar surface. Infection must be diagnosed clinically because, as with all skin wounds, polymicrobial flora is present, many of which are nonpathogenic. Systemic signs (e.g., fever, chills), purulent drainage, or at least two local signs of inflammation (e.g., calor [warmth], rubor [redness], dolor [pain, or tenderness], and tumor [induration]) are suggestive. Deep infections may have few surface signs. Chronic wounds may manifest discoloration, friability, delayed healing, or be malodorous. Signs of systemic toxicity are uncommon in DFi, even with limb-threatening infection, but metabolic abnormalities (e.g., hyperglycemia, ketoacidosis, hyperosmolar state) imply severe disease. Many patients do not complain of pain, and more than one-half of patients have neither fever, nor leukocytosis, nor an elevated erythrocyte sedimentation rate. Whenever the diagnosis of DFi is considered, aggressive management is indicated because these infections may progress rapidly.
Several severity scores have been proposed for DFi,43 but none has universal acceptance. Severity may be determined clinically by assessment of the depth of the wound and tissue perfusion. Local wound exploration with a sterile surgical instrument is important to identify necrotic tissue or the presence of a foreign body (e.g., a needle that the patient stepped on, but did not feel at the time) or osteomyelitis.44,45 Assessment of severity is essential for antibiotic selection (including route of administration) and to determine the need for hospitalization and the necessity and timing of surgical debridement or level of amputation. Systemic signs should raise suspicion of a deep infection. Indications for hospitalization include correction of hypovolemia or metabolic abnormalities, parenteral antibiotic therapy, or the need for surgery. Other reasons to hospitalize a DFi patient include an inability or unwillingness of the patient to provide local wound care or to maintain non–weight-bearing status or likely noncompliance with outpatient oral antibiotic therapy. Antibiotic therapy alone cannot overcome suboptimal wound care and inadequate glycemic control.
Acute DFIs are usually caused by gram-positive cocci; S. aureus is the most important pathogen in DFi. Whereas S. aureus is often present as a monomicrobial infection42,46 (Table 43.5), it is also usually an important pathogen in polymicrobial infections. Chronic wounds, recurrent infections, and infections of hospitalized patients are more likely to harbor complex flora, including aerobes and anaerobes.47 Among gram-negative bacilli, bacteria of the family Enterobacteriaceae are common. Pseudomonas aeruginosa may be isolated from wounds that have been treated with hydrotherapy or wet dressings. Enterococci may be recovered from patients treated previously with a cephalosporin. Anaerobic bacteria seldom cause DFIs as the sole pathogen, but they may be isolated from deep infections or necrotic tissue.47 Antibiotic-resistant bacteria, especially MRSA, may be isolated from patients who have received antibiotics previously or who have been hospitalized or reside in long-term care facilities.
TABLE 43.5
PATHOGENS ISOLATED IN A CLINICAL TRIAL OF ANTIBIOTIC THERAPY OF DIABETIC FOOT INFECTION
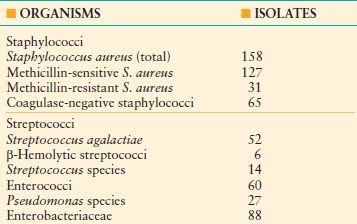
(50% of cases had only gram-positive cocci isolated).
Data from Lipsky BA, Itani K, Norden C, et al. Treating foot infections in diabetic patients: a randomized, multicenter, open-label trial of linezolid versus ampicillin-sulbactam/amoxicillin-clavulanate. Clin Infect Dis. 2004;38: 17–24.
Antibiotic Therapy. Forty to sixty percent of diabetic patients who are treated for a foot ulcer receive antibiotics, but antibiotic therapy does not improve the outcome of un-infected foot lesions in diabetic patients.39,48 Successful antibiotic therapy requires a therapeutic concentration of antibiotic at the site of infection. Intravenous antibiotics are indicated for patients with systemic illness, severe infection, intolerance of oral antibiotics, or pathogens that are not susceptible to oral agents. After the patient stabilizes and shows signs of improvement, a switch to oral antibiotic therapy may be appropriate. Slower delivery of antibiotic after an initial oral dose is inconsequential for non–critically ill patients, and so the main consideration is the bioavailability of the chosen agent. Among the potential choices of oral antibiotics for DFIs, clindamycin, linezolid, and fluoroquinolones have good oral bioavailability. Even parenteral antibiotics may not penetrate tissue adequately in the presence of peripheral arterial disease, even when serum concentrations are adequate.49
Initial empiric therapy should be directed at common pathogens (Table 43.5).46,48–51 Mild infections may be treated more narrowly because disease progression is unlikely to prevent modification of the regimen when microbiology data become available. For severe infections, regimens should utilize broad-spectrum intravenous antibiotics. Any regimen must account for allergy, renal function, recent antibiotic therapy, local susceptibility patterns, and the possibility of antibiotic-resistant pathogens. Empiric coverage for gram-positive bacteria (staphylococci and streptococci) is almost always required, and so the usual question is whether to broaden the regimen to cover gram-negative bacteria (Table 43.5).46,50,51 Anaerobic coverage should be considered for necrotic or malodorous wounds. If the patient responds to empiric therapy, the regimen may be narrowed according to microbiology susceptibility testing. The possibility of fastidious organisms missed by culture should be considered among nonresponders, or surgery may be needed.
Agents with demonstrated efficacy in clinical trials of therapy for DFIs include cephalosporins, β-lactamase inhibitor combination antibiotics, fluoroquinolones, clindamycin, carbapenems, glycopeptides, and linezolid (Table 43.6). The optimal duration of therapy for DFi has not been determined; common practice is to treat mild infections for 1 week, whereas serious infections that do not involve bone may require therapy for 2 weeks. Adequate debridement, resection, or amputation can shorten the necessary duration of therapy. Blood stream infection is a rare complication, for which many experts recommend a minimum of 2 weeks of therapy. Therapy may be discontinued when all signs and symptoms of infection have resolved; incomplete wound healing is not an indication to prolong antibiotic therapy.
TABLE 43.6
RECENT PROSPECTIVE TRIALS OF ANTIBIOTIC THERAPY FOR COMPLICATED SKIN AND SKIN STRUCTURE INFECTIONS OF THE FOOT IN PATIENTS WITH DIABETES MELLITUS
Adjuncts to Antibiotic Therapy. Hyperbaric oxygen (HBO) may improve wound healing and decrease the rate of amputation of DFi according to one double-blind, randomized trial.52 However, most evidence of HBO effect in DFi is anecdotal53 and difficult to interpret because of patient comorbidities that are poorly controlled for small sample sizes, and scant documentation of wound size and severity. Potential candidates for HBO therapy include patients at high risk for amputation (deep infections unresponsive to therapy). Objectively, HBO may be most beneficial when the transcutaneous oxygen tension is <40 mm Hg before therapy and increases to >200 mm Hg after therapy.53
Surgical revascularization may also be considered.54 Improving blood flow to the ischemic, infected foot may be a crucial determinant of outcome. Initial debridement is undertaken in the presence of infection; revascularization is generally postponed until sepsis is controlled, but should not be postponed more than a few days lest there be additional tissue loss in the meanwhile. Successful revascularization of an ischemic, infected foot can result in 3-year limb salvage rates of up to 98%.55
A good outcome may be expected in 80%–90% of mild cases treated appropriately and 50%–60% for more advanced DFIs. Aggressive surgical debridement is often needed for infections of deep tissue or bone. Partial amputations (e.g., toe amputation, “ray” amputation of a metatarsal) may be foot-sparing and lead to effective control of infection in >80% of cases. Healing is facilitated when there is no exposed bone, absent tissue edema, a palpable popliteal pulse, ankle systolic blood pressure >80 mm Hg, and a white blood cell count <12,000 per mm3. Infection recurs in 20%–30% of cases and should increase the suspicion of underlying osteomyelitis.
Osteomyelitis. Osteomyelitis is a feared complication of DFi (incidence, 50%–60% in serious DFIs; 10%–20% in mild infections).39 However, because diabetic patients may have destructive bone lesions caused by peripheral neuropathy (e.g., Charcot joint), distinguishing between neuropathic and infectious destruction of bone can be difficult. The likelihood of osteomyelitis is increased with foot ulcers that are chronic (>4 weeks), large (>2 cm diameter), deep (>3 mm), or associated with a marked elevation of ESR (>70 mm/h).39 Wound exploration that “probes to bone” has a positive predictive value of >90% for the diagnosis of osteomyelitis.
The initial diagnostic test should be plain radiographs of the foot. Radiographic changes may take 2 weeks to become manifest, and so repeating an initially negative study of a stable patient may be a better tactic than proceeding immediately to more sophisticated and expensive imaging. If clinical and plain radiographic findings are nondiagnostic, technetium 99m bone scans are 85% sensitive, but only 45% specific. Leukocyte scans (e.g., 111In) have similar sensitivity, but higher specificity (~75%). Magnetic resonance imaging (MRI) is usually the diagnostic test of choice because of high sensitivity (>90%) and specificity (>80%), but is expensive. A definitive diagnosis of osteomyelitis requires a bone biopsy for culture and histology. Bone biopsy must be obtained through a clean surgical incision that does not traverse an open wound to avoid contamination by colonizing organisms. Biopsy is indicated if the diagnosis remains doubtful after imaging studies, or if the etiologic agent(s) cannot be ascertained because of previous antibiotic therapy or confusing culture results. Most cases of osteomyelitis are polymicrobial; S. aureus is isolated most commonly (~40%), but S. epidermidis, streptococci, and Enterobacteriaceae are also common isolates.
Antibiotic therapy of osteomyelitis should be based on results of bone culture, because soft tissue culture results do not predict bone pathogens accurately.39 Empiric therapy should always cover S. aureus; broader coverage should be administered based on history or results of cultures. Most antibiotics penetrate bone poorly, and leukocyte function is impaired in these patients, and so long-term (at least 6 weeks) parenteral (at least initially) therapy is required. Osteomyelitis complicating DFi can be arrested by antibiotic therapy alone in about two-thirds of cases, and so resection of infected bone is not always necessary. Oral antibiotics with good bioavailability (e.g., fluoroquinolones, clindamycin) may be useful for most of the therapeutic course. If all infected bone is removed, a shorter course of therapy (e.g., 2 weeks) may be appropriate. Clinical resolution may be documented by a decrease to normal of the ESR or loss of increased uptake on a leukocyte scan.
SURGICAL SITE INFECTION
Infections of surgical incisions are now referred to as SSIs,56 a common surgical complication that occurs after about 3% of all surgical procedures.57 Potential complications of SSIs include tissue destruction, failure or prolongation of wound healing, incisional hernias, and occasionally blood stream infection. Recurrent pain and disfiguring scars may also result. Surgical site infections result in substantial morbidity, prolonged hospital stays, and increased direct patient costs, creating a huge economic burden on healthcare systems.58
Infection may occur within the surgical site at any depth, from the skin to the intracavitary operative field. Superficial incisional SSI involves tissues down to the fascia (Fig. 43.1), whereas deep incisional SSI extends into fascia and muscle, but not intracavitary. Organ/space infections are intracavitary, but, if related directly to an operation, are considered to be SSIs.
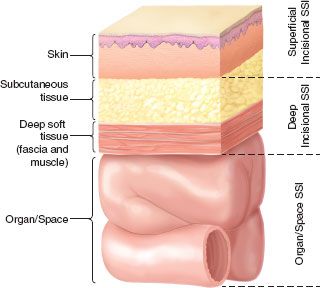
FIGURE 43.1. Cross-section of the abdominal wall depicting U.S. Centers for Disease Control and Prevention (CDC) classifications of surgical site infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








