Acute cerebral dysfunction is often the initial reason for presentation to the hospital and frequently develops as a complication of medical or perioperative care. The majority of causes require specific and urgent intervention, and understanding acute dysfunction of the brain is of paramount importance in leading the rapid and focused workup and guided therapies. Common disorders include ischemic stroke, intracerebral hemorrhage (ICH), subdural hemorrhage, subarachnoid hemorrhage (SAH), seizures, and encephalopathy (of infectious, inflammatory, hypo- or hypertensive, or toxic/metabolic origin). A timely and focused neurologic examination is critical in distinguishing focal versus generalized processes and can help to identify the likely etiology.
I. STROKE is the acute onset of a focal neurologic deficit or disturbance in the level of arousal due to cerebral ischemia, hemorrhage, or venous occlusion. Therapy is aimed at maintaining or acutely restoring adequate cerebral blood flow and preventing secondary brain injury.
A. Acute Ischemic Stroke is due to acute vascular occlusion. Symptoms often include sudden onset of visual loss, weakness or numbness on one side of the body, ataxia, unexplained falling, dysarthria, or aphasia. Thrombosis in situ may occur in diseased segments of small penetrating vessels (e.g., lacunar stroke) or larger arteries (e.g., atherosclerotic stenosis, arterial dissection), and emboli may be dislodged from proximal sites (e.g., heart, aorta, carotid artery) to lodge in otherwise normal major cerebral arteries or their distal branches.
1. Lacunar strokes tend to occur in patients with diabetes and chronic hypertension and may be clinically silent, but most often present (in descending order of frequency) as pure motor hemiparesis, sensory-motor stroke, ataxic-hemiparesis, pure sensory stroke, dysarthria-clumsy hand syndrome, or a variety of well-defined syndromes (e.g., hemichorea-hemiballism). As symptomatic lacunar stroke typically occurs in regions that interrupt major white matter tracts or brainstem nuclei, their initial presentation can be quite striking. However, the prognosis for recovery with lacunar stroke is better than with large-artery territory stroke. Nevertheless, because the risk of hemorrhagic transformation in these patients is low, many centers favor the use of intravenous (IV) thrombolysis in all but the most clinically mild lacunar strokes. Because initial small-vessel clinical syndromes may sometimes be due to large-artery thrombosis affecting end vessels, all patients presenting with acute ischemic symptoms should undergo some form of acute neurovascular imaging to establish large-vessel patency (e.g., computed tomographic angiography [CTA], magnetic resonance angiography [MRA], ultrasound, or conventional catheter angiography). Obtaining this imaging should not delay IV thrombolysis using recombinant tissue plasminogen activator (tPA, alteplase) in appropriate patients.
2. Large-artery occlusion is divided into disorders of the anterior (internal carotid artery and branches) and posterior (vertebrobasilar arteries and branches) circulations. These strokes carry a risk of swelling and hemorrhagic transformation. The “ischemic penumbra” refers to a region of brain with inadequate blood supply that still may be salvaged with rapid restoration of normal blood flow. Although the center of an ischemic zone (the core) may be irreversibly injured before the patient obtains medical attention, the surrounding ischemic penumbra may be saved by rapid intervention.
a. Middle cerebral artery (MCA) occlusion is characterized by weakness of the contralateral face and arm with hemianopia and a gaze preference toward the side of the involved hemisphere (“eyes looking toward the lesion”). Additional findings include aphasia in dominant-hemisphere strokes (the left hemisphere in the large majority of individuals), dense hemineglect in nondominant-hemisphere strokes (i.e., patient “ignores” the left side of the body, the surroundings, or the presence of the deficit itself; this is most often seen in lesion involving the R parietal lobe), and a variable degree of leg weakness depending on how much of the deep territory of the MCA is involved (and thus how much of the underlying white matter or basal ganglia is affected). Occlusion limited to branches of the MCA may produce partial versions of the MCA syndrome and are more likely to spare leg strength.
b. Anterior cerebral artery (ACA) occlusion in isolation is rare and causes isolated weakness of the lower limb. If both ACAs are affected, a generalized decrease in initiative (abulia) may also occur.
c. Border zone or “watershed” infarction is the result of insufficient blood flow to parts of the brain supplied by the distal territories of the major cerebral vessels. This develops most commonly in the setting of severe, sustained hypotension (e.g., cardiac arrest, profound shock prior to resuscitation) or in the presence of severe atherosclerotic narrowing of one or both carotid arteries. Because the region most commonly affected is the white matter underneath the motor areas (the ACA/MCA border zone), the classic presentation is that of proximal arm/leg weakness with preservation of distal strength, the so-called “man-in-a-barrel syndrome.”
d. Posterior circulation infarction involves the brainstem, cerebellum, thalamus, and occipital and mesial temporal lobes. As a result, patients can present with bilateral limb weakness or sensory disturbance, sensory and/or motor cranial nerve deficits, ataxia, nausea and vomiting, visual field deficits, or decreased level of consciousness, including coma. The full-blown syndrome results from occlusion of the majority of the basilar artery with fragments of the syndrome produced by occlusions of branches of the vertebrobasilar system. Edema and mass effect from cerebellar stroke may be life threatening due to confined space of the posterior fossa, with resulting upward or downward transtentorial herniation (see section on cerebellar hemorrhage).
3. Conditions mimicking stroke include seizure, migraine, toxic-metabolic derangement, and amyloid spells. Diffusion-weighted MR imaging helps to distinguish cerebral infarction from stroke mimics by identifying areas of intracellular swelling (i.e., cytotoxic edema) associated with ischemia and frank infarction.
a. While complex partial seizures may mimic stroke, especially if speech is impaired, postictal neurologic deficits (Todd’s phenomena) may masquerade as any focal neurologic deficit, including weakness, sensory loss, or aphasia lasting hours to days after a seizure.
b. The aura associated with a migraine headache may include focal neurologic deficits such as weakness, numbness, or aphasia and may occur in the absence of headache (“typical aura without headache”). Patients with recurrent migraine headaches are at a somewhat increased risk for true ischemic stroke. Patients who present with persistent symptoms similar in quality to their typical migrainous aura or who present with a new focal deficit accompanied by their typical aura should be evaluated for stroke.
c. Toxic-metabolic states such as hypo- or hyperglycemia, hyponatremia, hypoxia, or intoxication may produce focal or global neurologic deficits. Laboratory evaluation including rapid glucose evaluation (i.e., point-of-care glucose) and electrolytes should be performed in all cases. Occult infections can also exacerbate deficits from old strokes and brain injury and masquerade as new or recurrent stroke.
d. Patients with cerebral amyloid angiopathy may have transient neurologic dysfunction associated with microscopic hemorrhages that are suggestive of transient ischemic attacks (TIAs). Brain MR imaging with sequences sensitive to blood and blood breakdown products (i.e., gradient-echo or susceptibility-weighted imaging) may suggest a diagnosis of cerebral amyloid angiopathy.
4. Important etiologies of ischemic stroke include cardiac or arterial thromboembolism, intracranial and extracranial atherosclerosis, endocarditis, paradoxical emboli, arterial dissection, vasculitis, and inherited and acquired hypercoagulable disorders. Carotid or vertebral artery dissection may occur spontaneously, after trauma, or in connective tissue disease (e.g., fibromuscular dysplasia). Dissection can be recognized on axial T1 fat-suppression MR imaging or CTA or conventional angiography. Vasculitis may occur in primary central nervous system (CNS) disease or as part of a systemic syndrome such as systemic lupus erythematosus (SLE) or polyarteritis nodosa. Hypercoagulability may be due to clotting factor imbalance (e.g., protein C, protein S, or antithrombin III deficiency), autoimmunity (e.g., antiphospholipid antibodies), or inherited hypercoagulable states (e.g., prothrombin gene mutation). Sickle cell disease can also lead to focal cerebral arterial occlusion. In young patients with stroke, special attention should be given to the possibility of arterial dissection, hypercoagulable states, autoimmune syndromes, and hemoglobinopathies.
5. Acute evaluation for IV thrombolysis should be performed in all patients presenting within 4.5 hours of symptom onset to an appropriate facility. The only drug approved for use in acute ischemic stroke remains IV tPA. This includes accurate neurologic assessment, emergent CT (or MRI at some specialized centers) to exclude hemorrhage and early ischemic changes, laboratory exclusion of stroke mimics, hemostatic laboratories (platelets, prothrombin time [PT], activated partial thromboplastin time [aPTT]), electrocardiogram [ECG], and historical/imaging findings consistent with acute ischemia. Acute CT angiography (or if rapidly available MRA) of the head and neck can be very useful in selecting patients for catheter-directed thrombectomy. Specialized centers may offer endovascular approaches to reperfusion, including intra-arterial thrombolysis, mechanical thrombectomy, or angioplasty. These approaches may provide benefit beyond the 4.5-hour window of IV tPA, extending the window for acute intervention up to 6 to 8 hours in the anterior circulation, and perhaps up to 12 to 24 hours in the posterior circulation. Many therapeutic efforts to extend the window for acute intervention in ischemic stroke focuses upon the ischemic penumbra, that is, the region critically hypoperfused but potentially viable tissue around an irreversibly damaged core region of infarction. The penumbra can be imaged as a mismatch between perfusion-weighted magnetic resonance imaging (MRI) (PWI) and diffusion-weighted MRI (DWI) and is present in up to 80% of patients within 3 hours of symptom onset, although it diminishes rapidly with time. See http://www.acutestroke.com for the Massachusetts General Hospital Acute Stroke Service protocols and http://www.stroke-center.org for completed and active clinical trials in cerebrovascular disease.
6. Subacute evaluation should identify the cause and help define the risk for recurrent stroke. Transthoracic echocardiography (TTE) should be performed to exclude intracardiac thrombus and to assess left ventricular size and function, left atrial size, mitral and aortic valvular disease, and right-to-left shunt. In patients with suspected paradoxical embolism, agitated-saline contrast echocardiography should be performed to increase the sensitivity for the detection of shunt. Transesophageal studies are more sensitive to left atrial thrombus and atheromatous disease of the aortic arch. A 24-hour Holter monitor may identify paroxysmal atrial fibrillation, and if negative a 30-day event monitor increases the sensitivity for detecting atrial fibrillation. Particularly in young patients, the cause of the stroke should be vigorously pursued, including evaluation for inherited or acquired hypercoagulable syndromes.
7. TIAs are traditionally considered to be sudden, focal neurologic deficits that last less than 24 hours and are believed to be of vascular origin (Fig. 30.2). This definition is falling out of favor because ischemic symptoms lasting more than several hours almost always are associated with evidence of infarction on advanced imaging techniques (diffusion-weighted imaging [DWI]), and occasionally symptoms lasting only a few minutes also have imaging that demonstrates infarction. Therefore, even transient symptoms consistent with ischemic injury should be evaluated as potential ischemic stroke, and the newest American Heart/American Stroke Association guidelines recognize that similar principles apply in the workup and secondary prevention of TIA and ischemic stroke. The ABCD2 risk factor stratification score after acute TIA has been developed to estimate the risk for stroke within 2 days after TIA, and some centers have developed a “TIA clinic” allowing for expedited neurological evaluation and workup of patients with TIA.
8. Acute treatment. If the time of onset is clearly established to be less than 4.5 hours and cranial CT excludes intracranial hemorrhage or well-established stroke, all patients with a significant persistent deficit and the clinical diagnosis of ischemic stroke are potential candidates for IV tPA. A 0.9-mg/kg (maximum 90 mg) dose is infused over 60 minutes with 10% of the total dose administered as an initial IV bolus over 1 minute. Contraindications to IV tPA are summarized in Table 30.1. In 2009, the AHA/ASA issued a science advisory about the use of IV tPA in the 3-to-4.5-hour time window; they are essentially similar to those in the standard 3-hour time window but have the following additional exclusion criteria: older than 80 years, any history of recent oral anticoagulant use, NIHSS >25, or those patients with a concomitant history of stroke and diabetes. Following IV tPA, no aspirin, heparin, or warfarin should be given for 24 hours. Patients with severe strokes (National Institutes of Health Stroke Scale [NIHSS] >20; Table 30.2) have a higher rate of hemorrhage after tPA; however, many centers favor treatment of these patients, given their otherwise unfavorable prognosis. Proximal artery occlusions are less likely to recanalize with IV tPA and are more likely to produce severe clinical deficits.
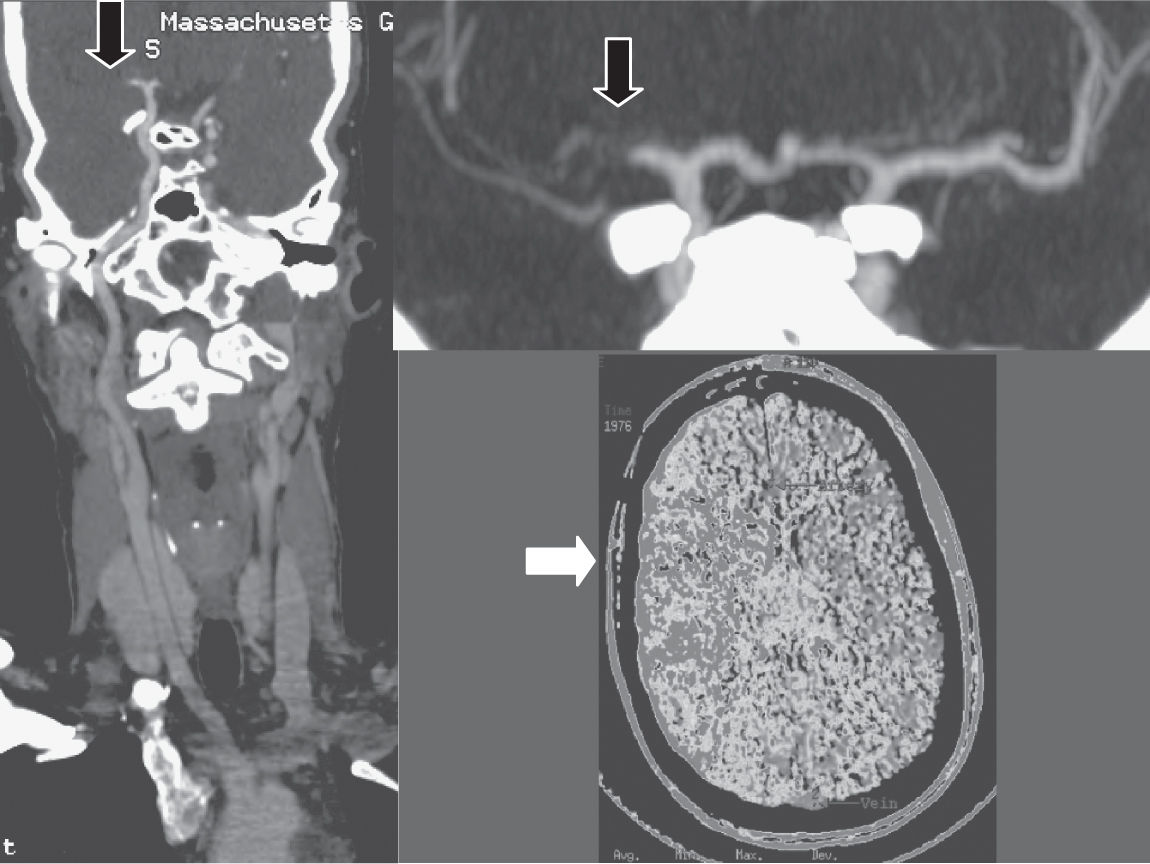
FIGURE 30.1 CT angiography three-dimensional formatted images in a 28-year-old female, demonstrating complete right MCA occlusion due to paradoxical embolism through a patent foramen ovale. The initial image shows a curved reformat from the aortic arch to the distal ICA bifurcation. The second image is a magnified view of the MCA stem occlusion. The third is a CT perfusion image showing abnormal perfusion to the right hemisphere in the territory of the occluded artery.
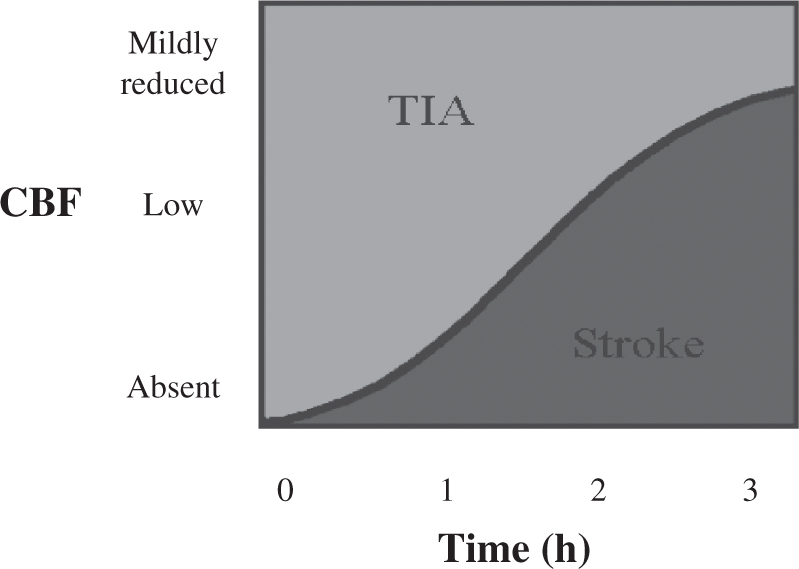
FIGURE 30.2 Graphic representation of cerebral ischemia as a function of both the degree of cerebral blood flow reduction and the duration of ischemia. Relatively mild reductions in blood flow can be tolerated for hours without progression to infarction, whereas steep reductions are poorly tolerated even for less than 1 hour.
| MGH Inclusion and Exclusion Criteria for Administering Intravenous Tissue Plasminogen Activator to Adult Patients with Acute Ischemic Stroke | |
Inclusion Criteria
• A significant neurologic deficit expected to result in long-term disability
• Noncontrast CT scan showing no hemorrhage or well-established new infarct
• Acute ischemic stroke symptoms with the patient last known well, clearly defined, <3 h before rt-PA will be given (Note: For additional warnings for the 3–4.5 h time period, see text.)
Contraindications
• SBP >185 or DBP >110 mmHg (despite measures to reduce it)
• CT findings (intracranial hemorrhage, subarachnoid hemorrhage, or major infarct signs)
• Platelets <100,000, PTT >40 s after heparin use, or PT >15 or INR >1.7, or known bleeding diathesis
• Recent major surgery or trauma (<15 d)
• Seizure at onset (if residual deficits are postictal impairments)
• Active internal bleeding (within prior 21 d)
• Recent intracranial or spinal surgery, head trauma, or stroke (<3 mo)
• History of intracranial hemorrhage or brain aneurysm or vascular malformation or brain tumor (may consider in patients with CNS lesions with very low likelihood of hemorrhage)
• Suspicion of subarachnoid hemorrhage (by imaging or clinical presentation)
• Current use of novel oral anticoagulants (NOACs) within 48 h, or with abnormal coagulation labs if >48 h since last dose
Warnings (conditions that might lead to unfavorable outcomes but not necessarily contraindications)
• Stroke severity too mild
• Rapid improvement
• Stroke severity—too severe (e.g., NIHSS >22; many centers do not exclude patients on the basis of an increased NIHSS alone)
• Glucose <50 or >400 mg/dL
• Life expectancy <1 y or severe comorbid illness or CMO on admission
• Increased risk of bleeding
• Subacute bacterial endocarditis
• Hemostatic defects including those secondary to severe hepatic or renal disease
• Diabetic hemorrhage retinopathy, or other hemorrhagic ophthalmic conditions
• Septic thrombophlebitis or occluded AV cannula at seriously infected site
• Patients currently receiving oral anticoagulants, e.g., warfarin sodium with INR >1.7
• Increased risk of bleeding due to pregnancy
• Advanced age (increased risk of bleeding)
• Documented left heart thrombus or recent MI (within 3 mo)
CT, computed tomography; NIHSS, National Institutes of Health Stroke Scale; INR, International Normalized Ratio; tPA, tissue plasminogen activator; CMO, comfort measures only. From the Massachusetts General Hospital Acute Stroke Services (www.acutestroke.com).

a. Mechanical clot retrieval has achieved renewed interest in the field since the publication of a number of randomized trials in late 2014–early 2015 with a newer generation of thrombectomy devices in conjunction with IV tPA and improved and timely patient selection with intracranial vascular imaging demonstrating a large artery anterior circulation thrombus. The first generation of devices was fed through the clot and meant to pull the clot out (Fig. 30.3). The newer devices used in these recent trials include temporary intra-arterial stents that are deployed into the clot and then retrieved or suction-aspirator devices that can break up and aspirate a clot in situ. There are multiple retrospective studies that demonstrate an association with worse neurologic outcomes in patient receiving general anesthesia for acute stroke therapies, and thus many centers are now preferring to treat patients with monitored anesthesia care or “conscious sedation” whenever feasible.
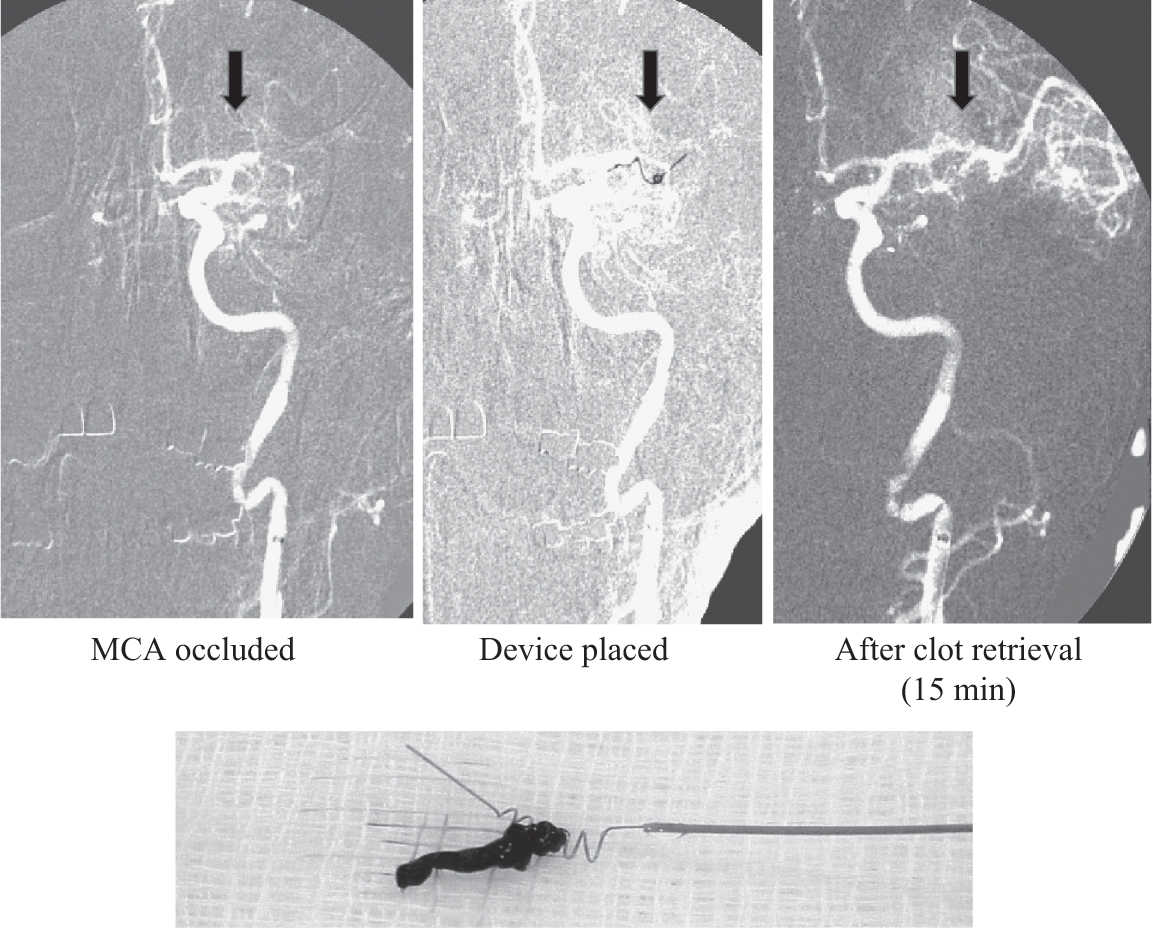
FIGURE 30.3 Serial angiographic images showing occlusion of the left middle cerebral artery, deployment of a clot retrieval device, and restoration of vessel patency. An example of residual thrombus is displayed below. The patient made a full recovery and was discharged several days later.
b. Continuous IV unfractionated heparin, although without proven benefit in acute stroke, is sometimes used in patients ineligible for thrombolysis and can be considered in patients with basilar stenosis, internal carotid or extradural vertebral artery dissection, fluctuating deficits, or symptomatic critical carotid stenosis without large MCA infarct. This use must be balanced against the risks of hemorrhagic complications. The aPTT should be monitored every 6 hours and the heparin dose adjusted accordingly. Because of high variability in individual heparin and aPTT assays, the aPTT should be maintained in the desired numerical range on the basis of the levels associated with achieving therapeutic anticoagulation (equivalent to 0.3–0.7 IU/mL by factor Xa inhibition), rather than a simple ratio of 1.5 to 2.5 times control. Initial heparin bolus may raise the risk of hemorrhage and is deferred except in fluctuating deficits or acute basilar thrombosis. While chronic anticoagulation reduces the risk of recurrent stroke in patients with atrial fibrillation, in patients with large infarcts, initiation is often deferred for days to weeks to minimize the risk of hemorrhagic transformation. Any patient who experiences a clinical deterioration on heparin must be imaged immediately to rule out hemorrhagic transformation.
c. Antiplatelet therapy should be considered for patients who do not qualify for thrombolytic therapy. Aspirin in doses ranging from 160 to 1,300 mg daily may benefit patients with acute stroke for whom thrombolytics or anticoagulants are not indicated, the AHA/ASA guideline recommends oral administration of aspirin 325 mg within 24 to 48 hours after stroke onset. Other antiplatelet agents such as IV glycoprotein IIb/IIIa inhibitors continue to be studied in acute ischemic stroke. Daily doses of aspirin commonly prescribed for secondary stroke prophylaxis range from 50 to 325 mg, with guidelines for coronary event prevention recommending a minimum dose of 75 mg daily. Combination aspirin (25 mg) plus extended-release dipyridamole (200 mg) has been shown to be superior to aspirin alone for secondary prophylaxis. Clopidogrel, another antiplatelet agent, is also useful for reducing the risk of recurrent vascular events and appears to be associated with less major bleeding than combination aspirin/dipyridamole in secondary stroke prevention. Dual antiplatelet therapy with the combination of aspirin and clopidogrel might be superior to aspirin alone when initiated within 24 hours of minor ischemic stroke or TIA for secondary stroke prophylaxis for the first 21 days, but because of increased risk of major bleeding complications it is not recommended for long-term secondary stroke prevention.
d. Urgent carotid revascularization may be indicated in cases of stroke in which there is a critical degree of carotid stenosis, a small distal infarction, and a large territory of vulnerable brain. Revascularization of larger strokes may be associated with acute reperfusion injury and should be delayed by weeks to months.
e. In some patients with stenosis of major vessels, pharmacologically induced hypertension with phenylephrine or other vasopressors may improve neurologic function acutely and rescue viable brain tissue, perhaps due to penumbral salvage. Early studies suggest that induced hypertension is safe in patients without cardiac comorbidities, such as angina or congestive heart failure. It is not clear which population will benefit from induced hypertension, and thus if it is attempted, a short period (30–60 minutes) of a modest (10%–20%) pharmacologic elevation with repeated neurologic exam should assess for potential improvement. If improvement is observed, it may be reasonable to continue modest induced hypertension with repeated neurologic evaluation and frequent (i.e., at least daily) to see if the patient continues to have a neurologic improvement with induced hypertension.
9. Subacute treatment. Hypovolemia and hyponatremia should be avoided, and intravascular volume should be maintained with isotonic solutions. Fever should be aggressively controlled because even mild hyperthermia worsens outcome. Swelling is maximal at 2 to 5 days after stroke onset, and standard increased intracranial pressure (ICP) management should be initiated (see Chapters 10 and 37). In massive hemispheric or cerebellar infarction, decompressive surgery can be lifesaving and may improve outcome.
B. Primary Intracerebral Hemorrhage (ICH). The broad differential diagnosis for intracranial bleeding includes ICH, epidural and subdural hemorrhage, SAH (described subsequently), venous sinus thrombosis (also described subsequently), and, rarely, isolated intraventricular hemorrhage. These can often be distinguished initially by noncontrast CT scan, though more advanced imaging (to be discussed) may be required. The most common locations for ICH are basal ganglia, thalamus, cerebral white matter, pons, cortical lobar surface, and the cerebellum. Long-standing hypertension is the most common cause (up to 75% of cases), although other etiologies are recognized, such as aneurysm, trauma, vascular malformations, cerebral amyloid angiopathy, coagulopathies, neoplasms, sympathomimetic drugs, septic emboli, and vasculitis. Metastases, especially adenocarcinoma and melanoma, may present with ICH or swelling. ICH as a primary process should be differentiated from hemorrhagic transformation of ischemic infarction, in which a bland ischemic stroke develops petechial bleeding or turns into a space-occupying hematoma.
1. Clinical syndromes. ICH often presents with headache, nausea, vomiting, and focal neurologic signs similar to those seen in ischemic strokes. The evolution of symptoms may occur more slowly than in ischemic stroke or may present as an acute and devastating condition. As a rule, patients with ICH present with systolic hypertension. In patients who were normotensive at baseline, this usually resolves over the first week; in chronic hypertensive patients, aggressive, multiple-drug treatment is often required to control blood pressure. In contrast to most cortical hemorrhages, the progression to death from cerebellar hemorrhage may be rapid.
a. Supratentorial ICH presents with symptoms referable to the site of bleeding. With rebleeding or development of vasogenic edema or hydrocephalus, there is often worsening of symptoms with decline in arousal. Transtentorial herniation is the mode of death in fatal massive lobar and basal ganglia hemorrhage.
b. Midline infratentorial hemorrhage produces only dysequilibrium on standing, walking, and sometimes sitting. Romberg sign cannot be assessed because balance is already impaired with eyes open. If gait is not tested, this lesion may not be detected until other cerebellar signs emerge secondary to brain swelling. Lateral cerebellar hemispheric lesions produce symptoms ipsilateral to the lesion. Patients complain of limb incoordination and demonstrate ataxia with falling toward the side of lesion, dysmetria (overshoot) on finger–nose–finger testing, dysdiadochokinesia (inaccuracy on rapid alternating movements), intention tremor (exaggerated on approaching the target), and nystagmus (worse looking toward lesion). Speech may be dysarthric (slurred), scanning, or explosive.
2. Acute evaluation of patients with suspected ICH consists of brain imaging; both CT and MR are very sensitive. In addition, toxicology screen, PT, aPTT, and platelets should be checked. Signs of occult malignancy should be excluded especially in patients without a history of hypertension. Hemorrhage volume correlates with outcome and can be estimated easily in cubic centimeters on unenhanced head CT using the “ABC/2” method, where A is the greatest diameter of the hemorrhage on a single slice, B is the hemorrhage diameter perpendicular to A on the same slice, and C is the approximate number of axial CT slices revealing hemorrhage multiplied by slice thickness in centimeters. The Intracerebral Hemorrhage Score was described over a decade ago and demonstrated that thirty-day mortality for patients with a parenchymal hemorrhage volume of greater than 60 cm3 on their initial CT and a Glasgow Coma Scale (GCS) score of 8 or less is 90%, and for those with a volume of less than 30 cm3 and a GCS score of 9 or greater it is 20%. The FUNC score is a recently validated clinical assessment tool that predicts, at hospital admission, functional independence at 90 days, and is available for use to clinicians (http://www.massgeneral.org/stopstroke/funcCalculator.aspx). Subacute evaluation should identify the etiology by imaging and history. MRI with gradient-echo or susceptibility-weighted imaging may identify areas of prior occult cortical hemorrhage and suggest a diagnosis of amyloid angiopathy in patients with lobar ICH. Repeat MR in 3 to 6 weeks may also detect lesions (e.g., tumor) masked by acute hemorrhage. Rarely, aneurysmal hemorrhage may result in primarily parenchymal hematoma, mimicking ICH. Conventional catheter angiography or CTA is indicated in any suspicious case. Prognosis is based on clinical presentation and imaging findings. Patients with cerebellar lesions less than 2 cm in diameter or with self-limited cerebellar signs usually do well, those with 3-cm lesions or progressive drowsiness do poorly without intervention, and 20% have lesions greater than 3 cm and a poor prognosis regardless of treatment. Prognosis in patients with cortical ICH is also related to hematoma size. It should be noted, however, that the most common cause of death in large ICH is withdrawal of supportive care, and the prognosis for large ICH with extended rehabilitation is less clear. Patients treated at hospitals that have a high rate of institution of DNR orders have higher mortality rates, and a recent trial of early full-aggressive medical care in patients with intracerebral hemorrhage demonstrated consistently lower mortality rates compared with the predicted mortality based on that predicted from prior ICH cohorts.
3. Acute treatment consists largely of supportive care, acute blood pressure control, reversal of coagulopathy, and ICP monitoring or surgical intervention in selected cases. To correct elevated PT, vitamin K, 10 mg infused at 1 mg/min, should be given IV, accompanied by rapid administration of prothrombin complex concentrate or transfusion of fresh frozen plasma (FFP); protamine is used for elevated aPTT associated with unfractionated heparin or low-molecular-weight heparin. Platelets should be provided to patients with platelet counts less than 100,000; patients who have uremic or pharmacologic (e.g., aspirin) platelet dysfunction may benefit from desmopressin. Reduction of systolic blood pressure to a target blood pressure of 160/90 is important to prevent rebleeding; If SBP is >200 mmHg or MAP is >150 mmHg, aggressive reduction of blood pressure with continuous IV infusion, with frequent blood pressure monitoring every 5 minutes is advised. If SBP is >180 mmHg or MAP is >130 mmHg and there is evidence of or suspicion of elevated ICP, then consider monitoring ICP and reducing blood pressure using intermittent or continuous IV medications to keep cerebral perfusion pressure >60 to 80 mmHg. Any clinical deterioration in association with reduction of BP should prompt reconsideration of ongoing BP management strategy. β-Blockers such as labetalol are preferred for blood pressure control, given their additional benefit of being antiarrhythmic; conversely, nitrates may paradoxically increase ICP by dilating the cerebral vasculature and are typically best avoided. IV calcium-channel blockers such as nicardipine may be useful if further reduction in blood pressure is needed. Neurosurgical consultation should be obtained early, especially in cerebellar hemorrhage of diameter 2 cm or greater, and an emphasis on surgical decompression and evacuation of hematoma should be considered (and not just placement of an extraventricular drain in cerebellar hemorrhage as this may promote upward transtentorial herniation). Resection of lobar or basal ganglia ICH can be life saving but has not been well studied in large clinical trials; the large randomized international trial only included patients in whom the treating neurosurgeon felt there was clinical equipoise (leading to the exclusion of a large number of patients at certain treating centers that favored early surgical hematoma evacuation). Surgical methods include open craniotomy and stereotactic drainage. Intraventricular tPA may also improve outcome in patients with intraventricular extension of the ICH. Obstructive or communicating hydrocephalus may develop and usually requires external ventricular drainage, although it may not need permanent ventricular shunting (Fig. 30.4). Corticosteroids do not appear to be of benefit in ICH. Anticonvulsant therapy is indicated in patients with clinical seizure but not for prophylaxis.
4. When ICH is suspected in patients who received thrombolysis for acute stroke, a head CT should be obtained immediately, along with neurosurgical and hematologic consultation, PT, aPTT, complete blood count (CBC), and D-dimer and fibrinogen concentrations. Treatment of verified symptomatic hematoma includes use of 2 U of FFP to replete factors V and VII, 10 U of cryoprecipitate to replete fibrinogen, and 6 U of platelets. Patients treated with heparin should receive protamine by slow IV push, 1 mg per each 100 units of unfractionated heparin given in the preceding 4 hours. If an anticoagulant dose of low-molecular-weight heparin had been used, the maximum dose of protamine (50 mg) should be given as slow IV push. The foregoing laboratory values should be repeated every hour for the next 4 hours until bleeding is brought under control. If these measures fail to control bleeding, aminocaproic acid, 5 g IV over 1 hour, may be given.
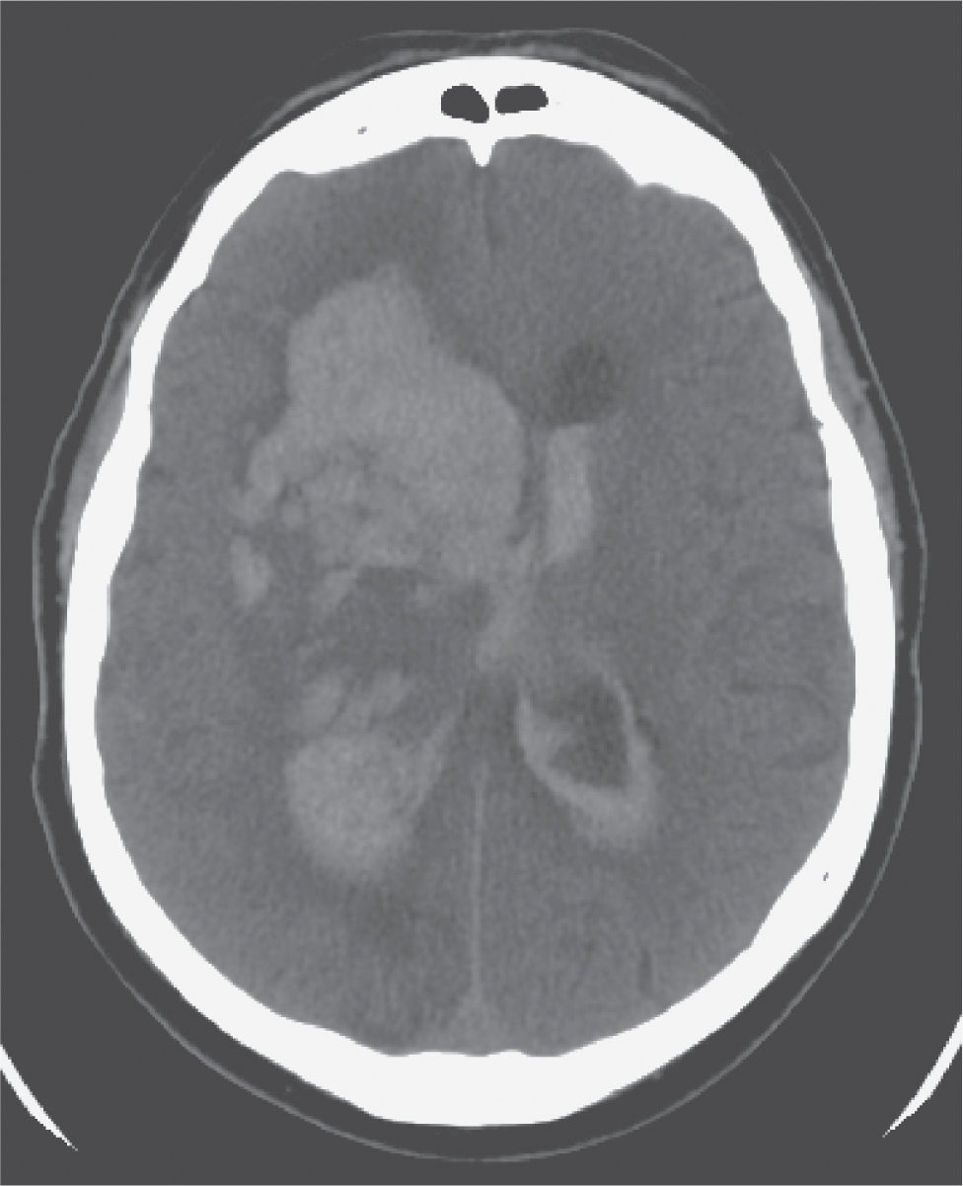
FIGURE 30.4 Axial image from an unenhanced CT of the brain showing a large right intracerebral hemorrhage with extension into the lateral ventricles and early obstructive hydrocephalus requiring ventriculostomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









