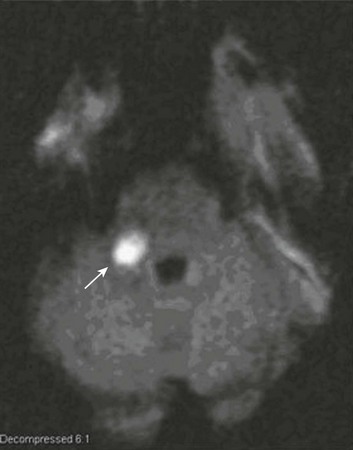
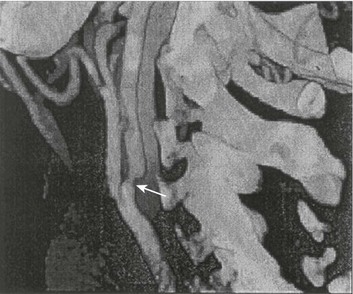
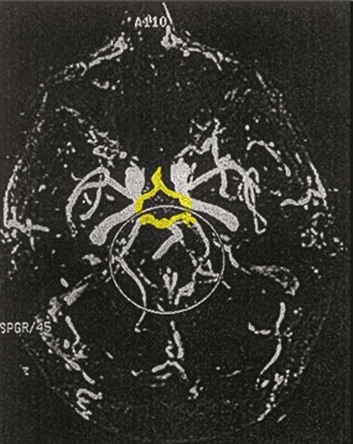
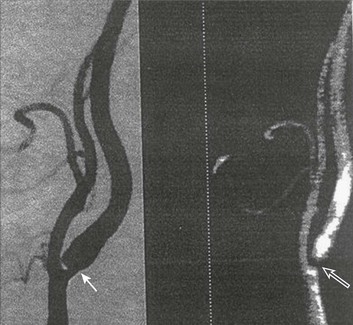
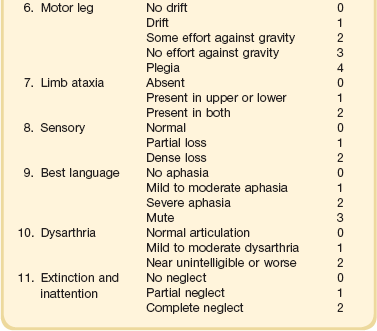
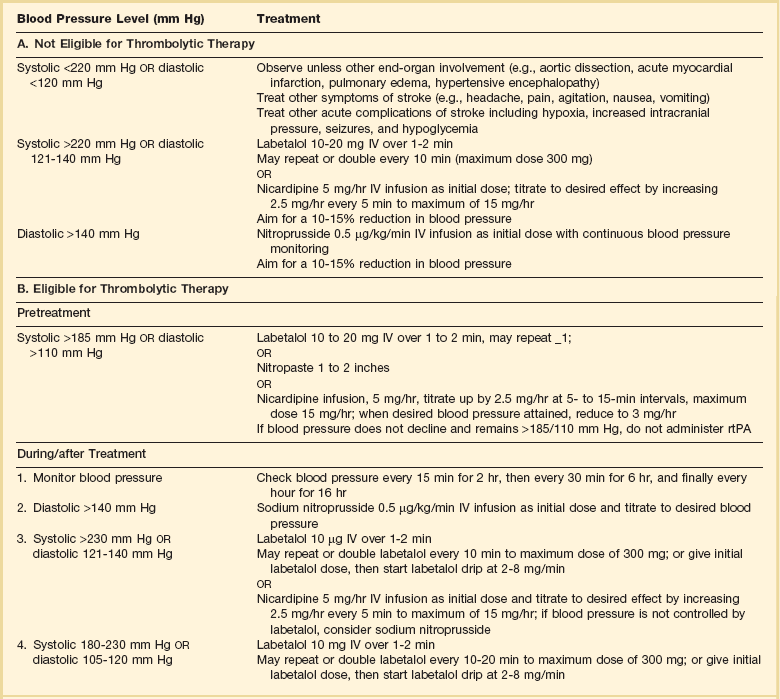
63 Stroke has a major impact in the United States, with an estimated yearly incidence of 731,100 new and recurrent strokes1 from 1993-1994. In 1997, 821,760 stroke admissions occurred in this country.2 Stroke constitutes the third leading cause of death and is a major cause of disability.3 Although stroke is a lesser cause of disability than heart disease,4 the population of stroke survivors continues to increase, in part because of a fall in mortality rate.5 Historically, stroke was not emphasized in the critical care setting because of the limited scope of interventions in the past. In the early 1990s, neurologic diseases, including but not limited to stroke, accounted for a mere 6% to 7% of admissions to critical care units.6 Now there is acute treatment for stroke, namely, the use of tissue plasminogen activator (tPA) for ischemic stroke within 3 hours of symptom onset,7 as well as intra-arterial (IA) thrombolysis for as long as 6 hours following stroke onset.8 A clot removal device (mechanical embolus removal in cerebral ischemia, or MERCI)9 has been approved, and newer, likely more effective, devices10 may become available shortly. Thrombolytic agents designed to work within a 9-hour time window, in conjunction with sophisticated imaging, have being studied, although they have not yet been shown to be useful.11 The great danger with the use of these agents is the risk of intracerebral hemorrhage (ICH). Frequent monitoring, as often as every 15 minutes following administration of a thrombolytic, is standard for patients so treated, and observation in a critical care unit is required. Consequently, critical care physicians need to learn about this condition, which has become a regular part of their professional lives, particularly in centers that devote themselves to the care of stroke patients. In one critical care unit with which the author is familiar, ischemic stroke accounts for 3% of the primary admissions and hemorrhagic stroke for 5.4%.12 Stroke is traditionally defined as a focal neurologic deficit of presumed vascular onset, lasting 24 hours or longer, as opposed to transient ischemic attack (TIA), which is an episode shorter than 24 hours in duration.13 Many TIAs actually last for less than 60 minutes.14 Carotid symptoms, as listed in Box 63.1, primarily consist of hemisensory loss, hemiparesis, and retinal ischemia (monocular blindness). Left hemispheric ischemia, generally in the perisylvian area, may result in varying degrees of aphasia.15 Involvement of the sensory association areas within the right parietal lobe can produce the phenomenon of neglect.16 In neglect, a stimulus is felt when it is alone but not in the presence of a competing stimulus. For example, a touch on the left hand or an object in the left visual field may be perceived when alone, but not when another stimulus is simultaneously presented, generally on the right side (double simultaneous stimulation). In that instance the right-sided stimulus alone is perceived. In extreme circumstances, affected individuals may not recognize the left side of the body as being theirs (anosognosia), as described memorably by Oliver Sacks in The Man Who Mistook His Wife for a Hat.17 Box 63.1 lists symptoms resulting from ischemia in the vertebrobasilar territory, which includes the cerebellum, brainstem, and the medial aspect of the occipital lobe, as well as the thalamus and the inferomedial portions of the temporal lobe. As a result, vertebrobasilar ischemia can produce cranial nerve dysfunction, nystagmus, cerebellar dysmetria, ataxia, and long tract signs such as sensory loss or motor impairment. These may involve one or both sides of the body. Memory disorders and visual field deficits also occur. In extreme circumstances, when the basilar artery becomes occluded, coma or quadriparesis may develop, although the presentation may vary, as described by Kubik and Adams18 in 1946. As a result of coma or quadriparesis, mechanical ventilation may be required, and the prognosis in such patients is grim. In one study,19 22 of 25 patients died, and the other 3 lingered in the “locked-in syndrome.”20 This frightening manifestation of basilar occlusion, secondary to pontine infarction, leaves patients chronically limited to eye blinking as their sole means of communication. Likewise, one must not label complaints that are not cerebrovascular in nature as stroke (see Box 63.1). Syncope, wooziness, and the like usually reflect systemic hypotension as opposed to focal ischemia. The still widespread practice of studying carotid vessels—most often through ultrasound—following the development of syncope should be abandoned because syncope does not result from ischemic stroke. In Box 63.1, item 4 under “Symptoms Not Considered Vascular in Origin” draws attention to the fact that certain symptoms may represent stroke when associated with other symptoms but not in isolation. Vertigo, for instance, can result from disease of the semicircular canals. If other complaints or findings referable to the posterior fossa of the brain (brainstem and cerebellum) coexist, such as those listed in Box 63.1 under “Vertebrobasilar Distribution,” the symptoms may indeed localize there. Similarly, amnesia alone may follow a seizure or result from transient global amnesia as opposed to stroke, and so on. The items listed in Box 63.2 reflect the presence of seizures. Seizure onset may be unwitnessed, and in the hospital only postictal deficits, such as aphasia or hemiparesis, may be observed. The appearance of any number of positive phenomena will draw attention to the correct diagnosis. These phenomena contrast with the abolition of normal function that happens with stroke and instead represent abnormal activity resulting from uncontrolled electrical discharges. Occasionally, however, limb shaking may represent carotid ischemia, usually as the result of hemodynamic compromise in the territory of the ipsilateral carotid artery.21 Dreifuss22 gives a comprehensive classification of epilepsy types and symptoms. Migraine can also be associated with focal neurologic complaints. Commonest among these are visual complaints including scotomas, whether scintillating or not. The most dramatic manifestation is hemiplegic migraine, which raises the fear of ICH at first presentation. Aphasia and paresthesia are also described. Silberstein and colleagues23 have reviewed the manifestations of migraine. Box 63.3 lists those entities that most frequently mimic stroke. Hypoglycemia may produce focal neurologic deficits. Occasionally a mass lesion such as tumor or subdural hematoma may present with fluctuating deficits or be revealed by seizure activity that may be confused with stroke. In the case of subdural hematoma in the elderly, the inciting trauma may have been minor or forgotten. Headache may be prominent, mild, or even absent. Mass lesions and hemorrhage are frequently marked by confusion, decreased level of consciousness, or headache, but if the lesion is small, these symptoms may not appear. Consequently, blood sugar measurements and computed tomography (CT) scans (without contrast) are obligatory in all instances of suspected stroke. Within the first 6 hours of the event, CT may well be negative, even in instances of major infarction such as that involving the entire MCA watershed. Thus CT finds its greatest utility not in confirming the clinical diagnosis but in excluding the presence of small hemorrhages. Neurologists have relied on clinical findings to diagnose stroke, especially in the hyperacute phase (0 to 6 hours), when CT is least helpful. With the advent of diffusion-weighted imaging (DWI), magnetic resonance imaging (MRI) scans (Fig. 63.1) can now be used to detect acute cerebral ischemia within the initial 6-hour period following symptom onset.24 This allows the clinician to confirm or exclude the presence of stroke in doubtful cases. Most commonly, such circumstances involve the possibility of a new lesion in a previously injured area of the brain. For example, in the case of a new seizure originating from the hemisphere affected by a prior stroke, DWI can show whether the new event is seizure alone or caused by a new stroke. By the same token, if a newly delirious or febrile patient should manifest worsening of a preexisting neurologic deficit, DWI will clarify whether the worsening stems from the intercurrent injury or from a coincident new stroke. DWI can also reveal silent areas of cerebral ischemia, which sometimes appear simultaneously with the area of injury that presents symptomatically as stroke. The coincident development of ischemia in different vascular territories may indicate the presence of an unusual mechanism of infarction, such as vasculitis, hypercoagulable state, or cardiac source of emboli. MRI has been proved to be just as effective as CT in detecting ICH,25 thus potentially removing an extra step (the initial head CT) from the stroke evaluation. Unfortunately, MRI is less immediately available than CT, requires more time and cooperation from the patient, and may not be feasible in the face of claustrophobia or of ferromagnetic implants/fragments within the body. Both CT and MRI technology can delineate the cerebral vasculature in detail, starting from the aortic arch and extending to the vicinity of the circle of Willis. Computed tomographic angiography (CTA) has been shown to be reliable in studying the intracranial vasculature26 and the extracranial segment of the carotid artery (Fig. 63.2).27 Magnetic resonance angiography (MRA) is superior to ultrasound in detecting carotid artery stenosis in the neck28 and is effective as well intracranially29 (Figs. 63.3 and 63.4), although its specificity and sensitivity in both instances are likely to improve. CTA has its own limitations, namely, the difficulty in performing the study in patients with contrast dye allergies. Because of these new techniques, the performance of conventional cerebral angiography is limited to specific indications, as outlined in Box 63.4. The era of thrombolysis in acute stroke began with the publication of the NINDS (National Institute of Neurological Disorders and Stroke) tPA trial in 1995.7 This groundbreaking study was the first to demonstrate a beneficial effect of tPA when given to patients presenting within 3 hours of the onset of the event. Depending on the criteria used to determine favorable outcome at 3 months, roughly an additional 11% to 13% of subjects receiving tPA recovered with little or no disability. If one uses the National Institutes of Health stroke scale (NIHSS) (Table 63.1) score, a reliable measure30 to measure disability, the improvement was from 20% with minimal or no disability with placebo to 31% with tPA. This was counterbalanced by an increase in the rate of ICH, from 0.6% in the placebo group to 6.4% in the tPA cohort. Half the subjects in the placebo group who suffered ICH died, whereas the mortality rate from ICH in the tPA cohort was 2.9% (less than half). Hemorrhage following tPA use occupies the same area of the brain affected by the initial thrombosis in most but not all cases.31 Strict inclusion (Box 63.5) and exclusion (Box 63.6) criteria are applied to attempt to minimize the risk of ICH. The safety and efficacy of thrombolysis have not been analyzed in children. The time of onset of symptoms is taken to be the last time that the patient was seen to be normal. For example, an individual who went to sleep at 10 PM and awoke at 6 AM, immediately hemiplegic, will not qualify for tPA therapy. One who awoke at 4 AM, went back to sleep, and awoke again at 6 AM with stroke symptoms may be treated with tPA, but only up to 7 AM. A person who awoke at 6 AM, was briefly normal, and developed stroke symptoms at 6:05 AM may be treated until 9:05 AM. The blood glucose must be determined before initiating tPA therapy to avoid misdiagnosing hypoglycemia as stroke (see Box 63.3). A CT scan of the head is essential to look for a mass, most often an ICH or subdural hematoma. The CT must also be scrutinized for the presence of early ischemic changes, such as sulcal effacement, hypolucency within the brain, or loss of definition between structures within the brain, or for the presence of a hyperdense MCA, suggestive of thrombosis.32 Whether ischemic changes on CT predict a heightened risk of hemorrhagic transformation is controversial.31,33 However, CT findings appear more commonly among subjects with scans performed relatively late in the course of their stroke.33 Hence their appearance should prompt reevaluation of the time of onset of the stroke. Prior ICH, recent stroke, and hypertension at presentation are believed to increase the risk of sustaining ICH. Avoiding systemic bleeding, which may result in hypotension and worsening of the neurologic deficit, is also important. Extremes of blood glucose or the presence of a coincident seizure make the neurologic deficit seem worse than it is, rendering calculation of a risk-benefit ratio more difficult. Two blood tests must be checked before embarking on thrombolysis: a blood sugar, as mentioned earlier, and a platelet count. If the patient is on anticoagulant therapy, the prothrombin time/international normalized ratio or partial thromboplastin time must be available before deciding whether to proceed with treatment. If a patient is not known to be receiving anticoagulants at baseline, but the coagulation profile proves abnormal, the infusion must be stopped if thrombolysis is still ongoing at the time that the abnormal value returns. If a patient is receiving low-molecular-weight heparin, there is no rapid way of determining the degree of anticoagulation, and intervention must be withheld. Testing for the activity of factor Xa antagonists is not yet standardized, although a normal thrombin time may suggest that thrombolysis is safe.34 If the blood pressure is elevated (>180 mm Hg systolic or 110 mm Hg diastolic), a modest dosage of labetalol, 5 to 10 mg IV (intravenous), may be given and repeated if necessary, up to a maximum dosage of 40 mg. Nitropaste is a less exact alternative but has the advantage that it can be removed. A nicardipine drip may be employed as well, which constitutes a change from prior guidelines. If the blood pressure subsequently rebounds to an undesirable range, tPA should not be given. If the pressure should rise above 180 mm Hg systolic or 105 mm Hg diastolic subsequent to starting the tPA infusion, it is paramount to bring it down using IV infusions of antihypertensive agents (Table 63.2).35 In this context, it is worth emphasizing that blood pressure should be treated acutely only when there exists a specific indication for doing so, as defined in the table. Table 63.2 Approach to Elevated Blood Pressure in Acute Ischemic Stroke rtPA, recombinant tissue plasminogen activator. From Adams HP, del Zoppo G, Alberts MJ, et al: Guidelines for the early management of adults with ischemic stroke. Stroke 2007;38:1655-1711. Hypotension may develop, raising the concern of systemic hemorrhage. Of course, hypotension may have a variety of other causes. The most feared event following tPA use is the development of ICH. The presenting signs and symptoms appear in Box 63.7. Most are self-explanatory. Hypertension is a compensatory response to increased intracranial pressure (ICP), to maintain cerebral perfusion, and bradycardia occurs secondary to it. The steps to be taken with suspected ICH appear in Box 63.8. It is relatively uncommon for neurosurgeons to intervene on hemorrhages in the setting of tPA because of the risk of further bleeding into the surgical bed. Systemic hemorrhage is handled in a similar fashion, but transfusion may also be necessary. Platelets should be administered if the count is significantly decreased (<50,000 cells/µL). The benefit of tPA is greatest in the instances of the smallest vascular occlusions (lacunar, as opposed to cortical, infarcts). Older subjects with particularly severe strokes are most likely to have a poor outcome, but even in this group tPA remains beneficial overall.36 The sooner tPA is administered, the higher the likelihood of successful recovery, as shown by a meta-analysis37 of thrombolysis trials. A residual benefit of tPA exists, as far out as 6 hours from the ictus. Two studies38,39 that specifically examined treatment beyond 3 hours failed to show a benefit of administration of tPA within a 5- or 6-hour time frame. More recently, however, the ECASS-III (European Cooperative Acute Stroke Study III) trial40 established the utility of tPA administration in the 3.0- to 4.5-hour period following stroke onset. The inclusion and exclusion criteria are more restrictive than those for treatment within 3.0 hours (Boxes 63.9 and 63.10). Initially, there was some concern about the administration of tPA within the 3.0- to 4.5-hour window to subjects with a history both of prior stroke and of diabetes. A statistical analysis41 based on datasets from large registries suggests that neither factor predisposes to poor outcome in reality. No doubt there exists a subgroup of subjects presenting beyond 4.5 hours that remains amenable to treatment, but this group cannot yet be identified. Various authors have identified different predictors of ICH following treatment with tPA. Levy and colleagues42 identified the dosage of tPA given, the age of the subjects, and diastolic hypertension as pertinent factors. Larrue and colleagues31 also found age to heighten the risk of development of ICH, but not the degree of hypertension or the time to treatment. It has become clear that tPA can be given safely in the community43 and that complication rates of tPA use can be lowered to a satisfactory level by careful adherence to the exclusion criteria.44 IA thrombolysis can be used to treat acute ischemic stroke beyond the 3-hour time window for IV tPA. Basilar thrombosis has been treated as late as 24 hours after the onset of symptoms using this approach.45 Other subjects with occlusion of the proximal (M1 or M2) segments of the MCA have been successfully treated between 3 and 6 hours following stroke onset. The utility of this approach rests on the PROACT II (Prolyse in Acute Cerebral Thromboembolism II) trial,8 a study using a novel agent, prourokinase (pro-UK). In contrast to the IV tPA trial, aspirin was allowed in the first 24 hours following stroke onset and heparin was used acutely following pro-UK to prevent vascular occlusion. To enter the study, the upper age limit was 85 years and the minimum NIHSS score was 11.
Stroke
Historical Background
Overview
Advances in Radiology
Thrombolysis in Stroke
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Anesthesia Key
Fastest Anesthesia & Intensive Care & Emergency Medicine Insight Engine