Sodium, Potassium, and Magnesium
Lawrence J. Caruso
Janet M. Caruso
CASE SUMMARY
A 73-year-old woman with obstructive jaundice and a pancreatic mass presented for a Whipple procedure. Past medical history was significant for type 2 diabetes mellitus, hypothyroidism, hyperlipidemia, and hypertension. Medications included hydrochlorothiazide, levothyroxine, glyburide, and simvastatin. Preoperative laboratory studies were remarkable for serum potassium of 3.0 mEq per dL and serum creatinine of 1.4 mg per dL. Preoperative electrocardiogram (ECG) showed sinus rhythm with a right bundle branch block. The patient was taken to surgery, which was notable for a surgical duration of 4 hours and blood loss of 1.5 L. She received 3 units of packed red blood cells and 4 L of normal saline. She was also given 40 mEq of potassium chloride. Urine output during the case totaled 350 mL. Following extubation, she was transported to the surgical intensive care unit (SICU) where she was noted to have frequent premature ventricular contractions (PVCs). The ECG showed no evidence of ischemia. Her postoperative potassium level was 2.6 mEq per dL. She received 60 mEq of potassium over 3 hours but continued to have PVCs. Repeat potassium level was 2.9 mEq per dL, and magnesium was 1.4 mEq per dL. She received 4 g of magnesium sulfate over 2 hours and an additional 60 mEq of potassium chloride. Her PVCs subsequently resolved, and serum potassium and magnesium levels increased to 3.7 mEq per dL and 2.0 mEq per dL, respectively. The remainder of her postoperative course was uneventful, and she was discharged home on postoperative day 7.
Why Are the Major Cations Important in Anesthesia?
Sodium (Na+), potassium (K+), and magnesium (Mg2+) are the major cations in the body (see Table 32.1). Perioperative alterations in concentrations of these electrolytes are common and can cause significant morbidity and mortality. The etiology and management of these disorders, with emphasis on perioperative considerations, are critical to the practice of anesthesiology.
▪ SODIUM
Sodium is the primary extracellular cation and accounts for most of the extracellular osmolality (see Equation 1):
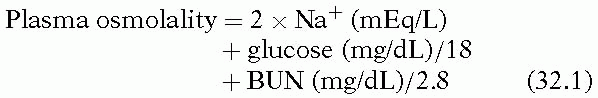
As a result, changes in Na+ levels can cause significant changes in serum osmolality and subsequent movement of water down the osmotic gradient. This movement of water, depending on the direction, causes cellular swelling or dehydration with associated morbidity.
What Is the Difference between Osmolality and Tonicity?
The concepts of osmolality and tonicity are often misunderstood. Osmolality, defined as the number of osmoles of solute per kilogram of water, is determined by the number of osmotically active particles in solution, whether they be permeant or impermeant to cell membranes. Tonicity refers to the number of osmotically active particles
that are impermeant to the cell membrane. Urea is an example of an osmotically active substance that is permeant to cell membranes. High urea concentrations will increase serum osmolality, but, because the urea moves freely across cell membranes, it does not cause hypertonicity and therefore does not cause movement of water across the cell membrane. Therefore, while hypoosmolal hyponatremia is always hypotonic, it is possible to have hypotonic hyponatremia with hyperosmolality (e.g., elevated blood urea nitrogen [BUN]).
that are impermeant to the cell membrane. Urea is an example of an osmotically active substance that is permeant to cell membranes. High urea concentrations will increase serum osmolality, but, because the urea moves freely across cell membranes, it does not cause hypertonicity and therefore does not cause movement of water across the cell membrane. Therefore, while hypoosmolal hyponatremia is always hypotonic, it is possible to have hypotonic hyponatremia with hyperosmolality (e.g., elevated blood urea nitrogen [BUN]).
TABLE 32.1 Approximate Electrolyte Concentrations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
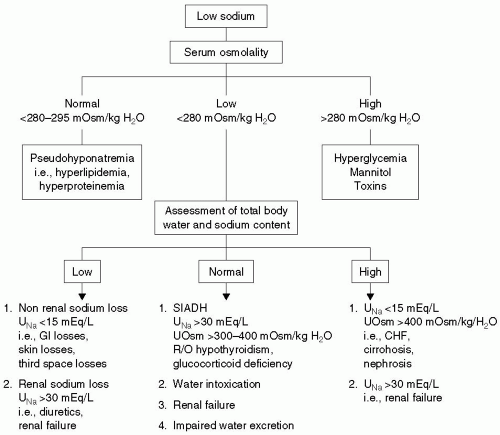
How Is Hyponatremia Defined and Classified?
Hyponatremia, defined as an Na+ level <135 mEq per L, can occur with normal, increased, or decreased serum osmolality. Hyponatremia can be classified on the basis of serum osmolality and intravascular volume status.
▪ HYPONATREMIA WITH NORMAL OSMOLALITY (PSEUDOHYPONATREMIA)
Normal plasma is made up of 93% water and 7% proteins and lipids. Increases in plasma protein (e.g., multiple myeloma) or lipids will decrease the relative amount of water in the plasma, and standard laboratory measurements (which measure plasma concentrations) will suggest hyponatremia. However, the concentration of Na+ in the aqueous phase of plasma remains normal; hence, the term, pseudohyponatremia. In reality, the changes of measured Na+ levels seen in these states are small (decrease of 1 mEq per L for each 500 mg per dL increase in triglycerides or 4 g per dL increase in serum protein).1 No treatment of the hyponatremia is required, although a search for the etiology of elevated protein or lipid is warranted.
▪ HYPEROSMOLAL HYPONATREMIA
Increases in serum osmolality (specifically, impermeable osmoles) will draw water from the intracellular to the extracellular space. This movement of water will dilute Na+ in the plasma, causing hyponatremia. Hyperosmolality can be caused by substances that are typically measured, such as glucose, or by unmeasured solutes (e.g., mannitol, ethylene glycol, glycine). The latter group will increase the gap between measured and calculated osmolality (i.e., osmolal gap, normal <10 mOsm per kg), whereas glucose elevations are associated with a normal osmolal gap. Ethanol, methanol, and propylene glycol are osmotically active and will increase the osmolal gap. However, these substances readily cross cell membranes, and therefore do not cause hyponatremia. A rule of thumb for the decrease in Na+ with hyperglycemia is that for every 100 mg per dL increase in glucose, the serum Na+ level decreases approximately 1.4 mEq per L.2
▪ HYPOOSMOLAL HYPONATREMIA
The vast majority of cases of perioperative hyponatremia are hypoosmolal. This entity can be further classified on the basis of the presence of hypovolemia, euvolemia, or hypervolemia.
Hypovolemic
Hypovolemic hypoosmolal hyponatremia is not uncommon in the perioperative period. This condition typically results from replacement of significant fluid losses with hypotonic fluids. Hypovolemia stimulates the release of antidiuretic hormone (ADH), which promotes water retention and further contributes to hyponatremia.
Surgical patients have a number of sources of isoosmotic fluid losses, both external and internal. External losses include hemorrhage; excessive urine output from diuretics or intrinsic renal disease; osmotic diuresis from mannitol, hyperglycemia, or radiographic contrast administration; or increased gastrointestinal (GI) losses from vomiting, diarrhea, fistula output, and nasogastric suction. Internal losses are commonly referred to as third space losses. These include losses into the interstitial space, the bowel lumen, and the peritoneal cavity. In certain situations, such as extensive tissue trauma, large burns, pancreatitis, and peritonitis, third space losses can be several liters. As a general rule, these losses should be replaced with isotonic solutions.
Cerebral salt wasting is a fairly common cause of hypovolemic hyponatremia in patients with neurologic injury, particularly those with subarachnoid hemorrhage. Although the mechanism is not well understood, elevated levels of natriuretic peptides cause significant renal Na+ and water losses, leading to hyponatremia and hypovolemia.3,4,5 Urine Na+ and urine osmolality are elevated and serum osmolality is low. These findings are also present in the syndrome of inappropriate antidiuretic hormone secretion (SIADH), and distinction between the two entities is difficult but important, as the treatment is markedly different.
Euvolemic
Euvolemic, hypoosmolal hyponatremia typically results from elevated levels of ADH, also known as arginine vasopressin. ADH is synthesized in the hypothalamus and released from the posterior pituitary gland in response to increases in plasma osmolality or decreases in effective circulating blood volume. It stimulates reabsorption of water in the collecting ducts of the kidneys, thereby increasing blood volume and decreasing plasma osmolality. This water is distributed throughout the body. Volume expansion and edema are not clinically evident, and intravascular volume regulation remains intact.
The kidneys normally have a remarkable ability to maintain normal osmolality over a wide range of fluid intake. A typical daily solute load is 1,000 mOsm. In the absence of ADH, maximally dilute urine of 50 to 100 mOsm per kg can be excreted, allowing excretion of up to 20 L of fluid per day. In the face of maximal ADH stimulation, urine concentration can be as high as 1,200 mOsm per kg. This capability of increased urinary concentration allows excretion of <1 L of fluid per day.
In addition to decreased blood volume and increased osmolality, a number of other factors increase ADH secretion, including pain, emotional stress, positive-pressure ventilation, nausea and vomiting, hypoxia, and hypercapnia. Several of these factors are often present in the perioperative period and can cause hyponatremia as a result of the elevated ADH levels.6,7,8,9,10,11,12 Chung et al. reported a series of surgical patients in which 4.4% developed a plasma concentration below 130 mEq per L within a week of surgery. Most of these cases were mild and were not associated with neurologic deterioration.13 Other investigators have reported more severe cases of postoperative hyponatremia with fatal cerebral edema.14
An increase in ADH levels in the absence of osmotic or hemodynamic stimuli is considered SIADH and is a diagnosis of exclusion. In addition to the common perioperative factors listed earlier, causes of SIADH include malignancy, pulmonary disease, central nervous system (CNS) disorders, and medications (see Table 32.2).
Other causes of euvolemic hyponatremia include psychogenic polydipsia, heavy beer drinking, renal failure, and diuretics, particularly thiazides. These diuretics block Na+ reabsorption in the distal convoluted tubule and prevent formation of maximally dilute urine.
Hypervolemic
Hypervolemic, hypoosmolal hyponatremia occurs in the setting of edematous states such as congestive heart failure (CHF), cirrhosis, nephrotic syndrome, and advanced renal failure. Total body water (TBW) and Na+ content are increased, but the increase in TBW is proportionally greater, leading to hyponatremia. Edema is typically present. In the case of CHF and cirrhosis, Na+ and water retention are stimulated by decreased circulating blood volume. With renal failure, excretion of Na+ and water are impaired.
What Are the Clinical Manifestations of Hyponatremia?
Clinical manifestations are largely dependent on the tonicity of plasma and the rapidity with which the changes in tonicity occur. Hyperosmolal, hypertonic hyponatremia causes a shift of water from cells to the extracellular space, leading to cellular dehydration. In addition to thirst, these patients generally have symptoms related to brain dehydration, including lethargy, weakness, seizures, and coma. Hypertonicity from toxic solutes may cause additional symptoms. For example, glycine absorption from prostatic irrigation fluid is associated with reversible blindness.
TABLE 32.2 Causes of Elevated Antidiuretic Hormone (ADH) Levels | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Most hyponatremia is hypotonic, causing movement of water into the cells. The main symptoms result from brain edema and include lethargy, confusion, coma, seizures, and weakness. In severe cases, cerebral edema advances to brain herniation. In acute hyponatremia, serum Na+ <120 mEq per L is associated with clinical manifestations and adverse outcome.15 If the onset is chronic, lower levels of Na+ will be tolerated, due to loss of intracellular organic osmolytes (previously termed, idiogenic osmoles) by brain cells over several days. Although these patients are less likely to be symptomatic, they are at risk for demyelination if the Na+ is corrected too aggressively (see Section, “How Is Hypernatremia Treated?”).
How Is the Patient with Hyponatremia Evaluated?
The initial approach to the patient with hyponatremia is to calculate the plasma osmolality by Equation 1:
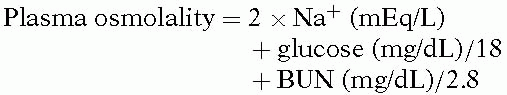
If the calculated osmolality is normal, no further evaluation is needed, and treatment is directed toward the causes of the elevated BUN, glucose, or both. If calculated osmolality is low, plasma osmolality should be measured. An increased measured osmolality (elevated osmolal gap) suggests the presence of unmeasured osmoles (e.g., mannitol, glycine, sorbitol), whereas a low reading that is similar to the calculated osmolality confirms hypoosmolality. Pseudohyponatremia (hyperlipidemia or hyperproteinemia) will present with a normal measured osmolality.
When hypoosmolal hyponatremia is confirmed, further evaluation is based on the patient’s volume status (see Fig. 32.1). Although hypervolemic hyponatremia is typically easily diagnosed on the basis of history and physical examination, it is often difficult to distinguish between hypovolemic and euvolemic hyponatremia. The clinical scenario is often helpful in distinguishing between euvolemic and hypovolemic hyponatremia. In the perioperative period, replacement of blood and fluid losses with hypotonic fluids (especially 5% dextrose in water) historically was a common cause of hypovolemic hyponatremia. In the presence of hypovolemia, the hemodynamic stimulation of ADH release overrides the tendency to excrete water to restore normal osmolarity. Because the current practice is to replace perioperative losses with isotonic fluids, this situation has become less common.
▪ DIAGNOSTIC MEASURES
Hypotension and tachycardia occur with severe volume depletion, but many other causes of tachycardia in the perioperative period, such as pain, anxiety, and drug withdrawal play a role. Elevated BUN and uric acid levels are suggestive of hypovolemia. Urinary Na+ levels may also be helpful. A urine Na+ <30 mEq per L in the presence of normal Na+ intake suggests hypovolemia. Patients with SIADH (euvolemia) typically have a urine Na+ >30 mEq per L. Urine Na+ measurement is not helpful in the context of salt-wasting disorders (Addison’s disease, cerebral salt-wasting, diuretics) because these patients will have high urine Na+ levels despite volume depletion. Therefore, volume status becomes the distinguishing feature between the hypovolemic salt-wasting syndromes and SIADH, which is euvolemic or slightly hypervolemic.
If volume status remains unclear, invasive monitoring may be necessary. Alternatively, the response to administration of isotonic saline may be helpful. In the patient with hypovolemia, volume expansion will decrease ADH levels, resulting in a less concentrated urine, whereas patients with SIADH (or salt-wasting syndromes) will simply excrete the extra Na+. As in all patients with hyponatremia, Na+ levels should be monitored closely during fluid administration.
How Is Hyponatremia Treated?
The treatment of hyperosmolar and isoosmolar hyponatremia is directed toward the underlying cause and may be as simple as awaiting excretion of mannitol or as involved as renal replacement therapy for uremia. For hypoosmolal hyponatremia, the treatment will vary, based primarily on the patient’s volume status. Patients with hypovolemia are managed with diuretics and attention to the underlying disease. In the patient with hypovolemia, volume repletion with isotonic fluids will decrease ADH levels and usually restore Na+ levels toward normal. Patients with salt-wasting syndromes typically require infusion of hypertonic saline. In severe or refractory cases, the mineralocorticoid effects of fludrocortisone or hydrocortisone can be used to reduce renal Na+ excretion.16,17
Patients with SIADH require fluid restriction, typically to 1,000 mL per day, along with management of the underlying cause of elevated ADH. While high salt intake limits the amount of free water and may be helpful, isotonic saline is ineffective in these patients as salt can be excreted in a higher concentration than it is administered, resulting in free water retention. Hypertonic saline is warranted only if the patient is symptomatic. The amount of 3% saline to administer can be calculated as follows:
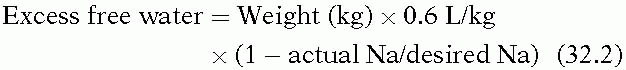
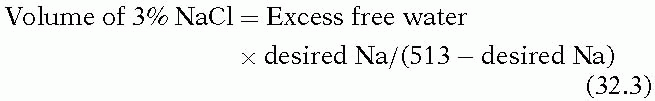
Rapid correction of chronic hyponatremia has been associated with central pontine myelinolysis (osmotic demyelination syndrome). Although it is controversial as to how rapidly hyponatremia can be safely corrected, it appears to be the absolute degree of correction that is important.18,19 A rational approach corrects symptomatic hyponatremia at an initial rate of 1 to 2 mEq/L/hour until symptoms resolve, at which point correction can proceed more slowly. Rapid, complete correction is unnecessary, and, in chronic hyponatremia, the Na+ should not be increased more than 25 mEq per L over the first 48 hours.
How and Why Does Hypernatremia Occur?
Hypernatremia, defined as a plasma Na+ > 145 mEq per L, represents an imbalance between total body Na+ and water content. As such, the condition may reflect excess Na+ with normal TBW—or more commonly, normal Na+ content with decreased TBW. Hypernatremia is always associated with hyperosmolarity, and its clinical features are mainly due to cellular dehydration: Thirst, weakness, and neurologic symptoms, ranging from lethargy to coma. With severe acute hypernatremia, brain shrinkage may lead to intracranial hemorrhage. Polyuria leads to volume depletion and may be severe enough to cause hypotension and tachycardia.
▪ PERIOPERATIVE FLUID LOSS
Several clinical scenarios are associated with perioperative hypernatremia. As was mentioned previously, perioperative fluid losses are typically replaced with isotonic fluids. When the fluid to be replaced is plasma, as in third space losses, replacement with isotonic fluids maintains normal plasma osmolality. However, many fluid losses, such as those from nasogastric suctioning and diarrhea, are hypotonic. Evaporation from the skin and respiratory tract accounts for a loss of 500 to 1,000 mL of water per day, and these losses increase in the presence of fever. Patients with burn injuries can have large evaporative losses from their wounds, as estimated by the following formula:
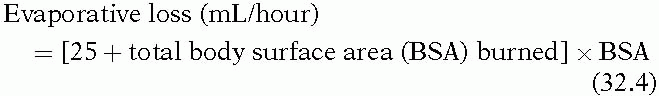
Replacement of these hypotonic losses with isotonic fluid may lead to hypernatremia, especially if the patient is hypovolemic and sodium-avid.
▪ ADMINISTRATION OF HYPERTONIC SOLUTIONS
Hypernatremia also results from the administration of hypertonic Na+ solutions. For the most part, these typically are hypertonic saline that is used to restore intravascular volume or decrease intracranial pressure, or sodium bicarbonate that is used to treat metabolic acidosis. Sodium bicarbonate ampoules have a concentration of 1 mEq per mL, or 1,000 mEq per L, approximately twice the osmolality of 3% sodium chloride.
▪ DIABETES INSIPIDUS
Another important cause of hypernatremia is diabetes insipidus (DI). As was discussed earlier, plasma osmolality is regulated by the release of ADH, which stimulates water reabsorption in the distal nephron. Two types of DI have been described: (i) Central DI, in which ADH release is absent or inappropriately low in the presence of osmotic stimuli; and (ii) nephrogenic DI, in which the kidneys are unresponsive to ADH.
Central DI is commonly seen after neurosurgery, particularly surgery involving the hypothalamus or pituitary gland. Severe traumatic brain injury is also associated with DI, and the development of polyuria may be an indicator of progression to brain death. Ethanol and phenytoin impair the central release of ADH.20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree