Chapter 10
Septic Shock
Clinical Considerations
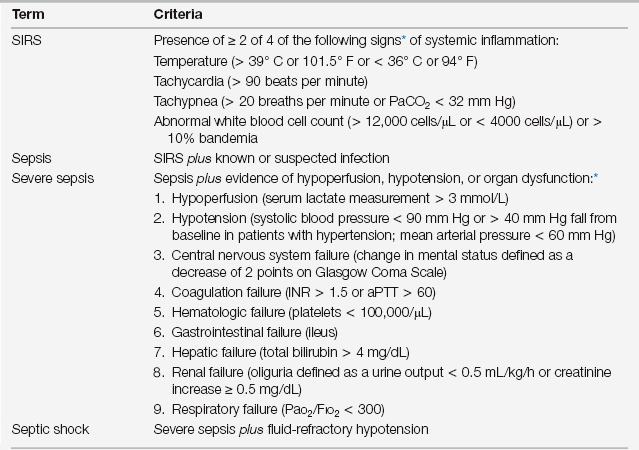
Definitions and Clinical Manifestations
The clinical manifestations of sepsis include the local symptoms and signs of the inciting infection as well as systemic signs, which are manifestations of the body’s response to the infection. Although the clinical features of the infection are relatively specific to the anatomic site, the systemic response is not. The systemic response, formally called the systemic inflammatory response syndrome (SIRS), includes both vital sign (fever, tachycardia, and tachypnea) and laboratory (leukocytosis or leukopenia) abnormalities (Table 10.1).
Consider sepsis severe when associated with hypoperfusion, organ dysfunction, or hypotension. Criteria for organ dysfunction have been specified (see Table 10.1). Septic shock is defined as refractory hypotension despite an adequate intravenous fluid volume challenge (> 20 to 30 mL/kg of crystalloid, or approximately 1 to 1.5 L). This categorization is important clinically, as it informs both triage and therapeutic decisions and has prognostic value.
Pathophysiology
TLRs also play an integral role in the initiation of the “cytokine storm” of sepsis. These inflammatory cytokines stimulate neutrophils and endothelial cells, including the activation of procoagulant pathways. This adaptive immune reaction, through a complex, interactive relationship, augments innate immune activation to enable a more effective response to the infection. Simultaneously activated biologic pathways down-regulate and control this response; however, the host’s immune response may become maladaptive, resulting in organ dysfunction, circulatory shock, and death. The specific mechanisms that regulate this response, and the genetics underlying their control, currently represent intense areas of investigation. Although the multifactorial pathophysiology of organ dysfunction extends beyond the scope of this chapter, circulatory shock plays a central role. Shock is an important and early clinical manifestation of severe sepsis, and treatment of shock may be lifesaving. Therefore, it is important to understand the pathophysiology and hemodynamics of septic shock.
Importantly, although widespread vasodilation (and low afterload) is a central feature of septic shock, the physical exam findings characteristic of a high cardiac output state (i.e., “warm shock”) are often absent on initial presentation. Rather, septic patients commonly present with findings consistent with a low cardiac output (i.e., “cold shock”), exhibiting a narrow pulse pressure, cool extremities, and mottled skin. This apparent paradox can be reconciled by dismissing the notion that vasodilation directly allows a reflex increase in cardiac output. A cardiac output rise in response to systemic vasodilation requires sufficient driving pressure (i.e., the mean systemic pressure; Pms) to the venous system to allow an increase in venous return, as cardiac output must equal venous return. Pms, as determined by the venous blood volume and compliance of the venous blood vessels, is often decreased on patient presentation because of profound hypovolemia and sepsis-induced venodilation (which increases venous compliance), respectively. Furthermore, SIC, present in many patients, further contributes to the impairment in cardiac output. This pathophysiology can lead to the findings of poor extremity perfusion on admission, as well as a low central venous pressure (CVP) and central venous oxygen saturation (ScvO2).
Differential Diagnosis
A limited set of disorders can mimic the warm shock (low afterload) state characteristic of well-resuscitated septic shock (Box 10.1); however, in those patients presenting in cold shock, one must consider a broader differential including disorders associated with a low cardiac output and high afterload. This broader differential includes all the other categories of shock including hypovolemic, cardiogenic, and obstructive. Although this includes a long list of disorders, initial findings on history and physical exam (e.g., clinical setting and absence of jugular venous distention and rales) usually allow one to readily exclude cardiogenic and obstructive shock from initial consideration.
Clinical Management of Septic Shock
Recognition
It is vitally important to recognize sepsis promptly, particularly when severe, as delays will reduce the effectiveness of lifesaving interventions. Early recognition may be challenging, particularly in the hospitalized patient, as clinical presentations can vary widely—and at times may be subtle—depending on the source of infection and host comorbidities. In sick patient populations, SIRS criteria have low diagnostic utility, as they are overly sensitive and nonspecific. In addition, in some particularly vulnerable populations such as the elderly or in those taking immunosuppressive medications, the clinical manifestation of both the infection and the SIRS response may be markedly attenuated despite overwhelming infection. In these patients, the signs of organ dysfunction (e.g., delirium or oliguria) may be the only clinical clue of underlying severe sepsis. This underscores the importance of maintaining a high degree of clinical suspicion for the presence of sepsis as the underlying cause of any clinical deterioration in the critically ill patient. Given the adverse impact of recognition delays on the effectiveness of some interventions, it is prudent to initiate treatment presumptively for severe sepsis, unless—or until—an alternative diagnosis has been established.
Diagnosis
Sepsis severity is readily established by physical exam and routine laboratory studies (see Table 10.1). Although some of these patients may be cared for on the general ward, when there is cardiovascular dysfunction, additional lifesaving interventions may be indicated. Specifically, septic shock or cryptic septic shock (lactate ≥ 4 mM/L, with a normal or high blood pressure) warrants early goal-directed therapy (EGDT) and prompt ICU transfer. Thus, a serum lactate should be obtained promptly in all patients with suspected sepsis, regardless of whether any other signs of organ dysfunction exist. In addition to high serum lactate levels (lactate ≥ 4 mM/L), intermediate lactate levels (i.e., ≥ 2 mmol/L) are associated with increased morbidity and mortality, independent of other organ dysfunction or level of blood pressure. The utility of serum lactate to risk-stratify the septic patient has been demonstrated across the continuum of care, from the pre-hospital environment to the emergency department to the ward and ICU patient. Conversely, the septic shock patient who maintains a normal serum lactate level throughout resuscitation appears to have a more favorable prognosis (Hernandez et al, 2012). Finally, in the proximal phase of resuscitation, transient hypotension (Marchick et al, 2009) and both abnormally low (< 70%) and abnormally high (≥ 90%) maximal ScvO2 measures (Pope et al, 2011) identify patients at risk of subsequent adverse events (e.g., mortality).
Antimicrobial Therapy
Timely administration of effective broad-spectrum antimicrobial therapy remains paramount in the initial management of the septic patient. Mortality increases nearly 8% for every hour delay in antibiotics administration beyond the first hour of hypotension. In addition, if the initial antimicrobial regimen is ineffective against the pathogen, mortality increases despite subsequent administration of appropriate antibiotics based on culture data. Therefore, empiric broad-spectrum antimicrobial therapy should be administered within 1 hour of identification of septic shock. Furthermore, in patients with severe sepsis, a delay in administering antimicrobial therapy until after shock recognition is associated with increased mortality (Puskarich et al, 2011).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree